
Michel Laurin, Jacques A. Gauthier, and S. Blair
Hedges *
Mammals, turtles, lizards, sphenodon, crocodiles,
birds, and their extinct relatives.
Phylogeny modified from Laurin and Reisz (1995) and Lee (1995). Node names follow Gauthier et al. (1988b) and Gauthier (1994).
Containing clade(s): Terrestrial Vertebrates
Amniotes include most of the land-dwelling vertebrates alive today, namely, mammals, turtles, Sphenodon, lizards, crocodylians and birds. It is a diverse clade with over 20000 living species. Amniotes include nearly all of the large plant- and flesh-eating vertebrates on land today, and they live all over the planet in virtually every habitat. They also sport disparate shapes - chameleons, bats, walruses, Homo sapiens, soft-shelled turtles, ostriches and snakes are but a few examples - and they include some of the smallest (sphaerodactyline geckoes) and largest (mysticete whales) vertebrates (Figs. 1 and 2). Although fundamentally land dwellers, several clades such as ichthyosaurs, plesiosaurs, pinnipeds and cetaceans have returned to the sea. A few forms are gliders - the Flying Dragon lizards, Honey Creepers, and Flying Squirrels - and powered aerial flight has originated three separate times, first in pterosaurs, then in birds, and later still in bats.


Figure 1. Two extant amniotes (diapsids). The rattlesnake can detect its prey at night using an infrared-sensitive organ that allows it to detect the warm body of small mammals. It then kills its prey with it poisonous fangs. Parrots eat nuts and fruits.


Figure 2. More extant amniotes. The insectivorous elephant shrew (a synapsid) resembles vaguely the earliest placental mammals. The omnivorous terrestrial box turtle Terrapine (an extant anapsid) eats mushrooms, fruits, insects, and worms. Like all turtles, it does not age but eventually succombs to a disease, an accident, or a predator. Pictures by John Merck.
Many amniote synapomorphies are widely interpreted as adaptations to the rigors of life on land. Indeed, Amniota owes its name to what may be its most distinctive attribute, a large and hard-shelled "amniotic" egg, which possesses of a unique set of membranes: amnion, chorion, and allantois (Fig. 3). The amnion surrounds the embryo and creates a fluid-filled cavity in which the embryo develops. The chorion forms a protective membrane around the egg. The allantois is closely applied against the chorion, where it performs gas exchange and stores metabolic wastes (and becomes the urinary bladder in the adult). As in other vertebrates, nutrients for the developing embryo are stored in the yolk sac, which is much larger in amniotes than in vertebrates generally. Hatchling amniotes also possess an egg-tooth and horny caruncle on the snout tip to facilitate exit from their hard-shelled eggs. The amniotic egg, together with a penis for internal fertilization, loss of a free-living larval stage in the life cycle, and the ability to bury their eggs, enabled amniotes to escape the bonds that confined their ancestors' reproductive activities to aquatic environments. Some components of the amniotic egg have been variously modified within Amniota. Placental mammals, for example, have suppressed the egg shell and yolk sac, and elaborated the amniotic membranes to enable nutrients and wastes to pass directly between mother and embryo.
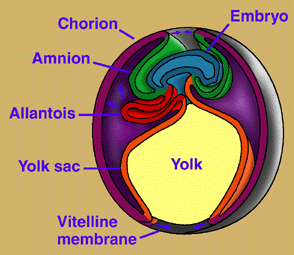
Figure 3. Development of extraembryonic membranes in an amniote egg (chick). In this early developmental stage, the yolk sac is expanding over the yolk. The amnion and chorion are expanding over the embryo and will eventually form the amniotic chamber. The allantois is expanding toward the chorion, with which it will form a respiratory membrane, in addition to storing metabolic wastes of the embryo. Redrawn from Campbell (1993).
Many of these features are rarely preserved in fossils, but there are some novelties in the skeleton that are no less diagnostic of amniotes. For example, amniotes have at least two pairs of sacral ribs, instead of just one pair. They also have an astragalus bone in the ankle, instead of separate tibiale, intermedium, and proximal centrale bones. Finally, they have paired spinal accessory (11th) and hypoglossal (12th) cranial nerves incorporated into the skull, in addition to the ten pairs of cranial nerves present in amphibians.
Generations of systematists have studied amniote phylogeny at diverse genealogical levels, and its broad outlines are reasonably well understood. Indeed, recognition of the major living clades, such as mammals, turtles and birds, antedates the Theory of Descent. Relations among these taxa, and especially the connections of various fossils to them, have been contentious in post-Darwinian times. Much of that controversy can, however, be attributed to the fact that during the first two-thirds of this century, there was little thought given to what constituted evidence for phylogenetic relationships. The origins of the major extant lines of Amniota have become clearer in the post-Hennigian era. Nevertheless, the precise relations of a number of clades, most notably the turtles among extant forms and the aquatic and highly divergent ichthyosaurs and sauropterygians among extinct forms, remain contentious.
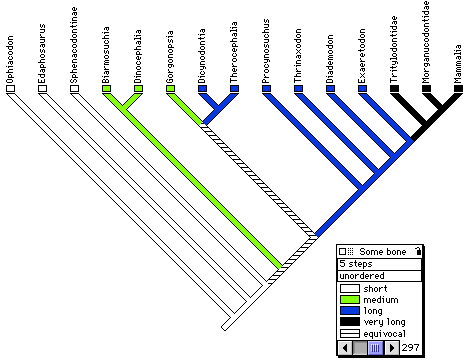
If extinct amniotes are considered, the phylogeny is much more complex and controversial. Formerly, captorhinids were believed to be closely related to turtles (Gauthier et al., 1988b, c), but more recently, procolophonids (Reisz and Laurin, 1991; Laurin and Reisz, 1995), pareiasaurs (Lee, 1993, 1994, 1995, and 1996), and even sauropterygians (a group of Mesozoic diapsids) have been suggested to represent early relatives of turtles (Rieppel, 1994, 1995). The linked page Phylogeny and Classification of Amniotes provides information about the phylogenies incorporating extinct amniote taxa, and provides a detailed classification of the relevant groups. The linked page Temporal Fenestration and the Classification of Amniotes discusses how temporal fenestration has been used to classify amniotes, and how tempororal fenestration evolved.
Bishop, M. J., and A. E. Friday. 1987. Tetrapod relationships:
the molecular evidence. In Patterson, C (ed.) Molecules
and Morphology in Evolution: Conflict or Compromise?:
123-139. Cambridge: Cambridge University Press.
Campbell, N. A. 1993. Biology. 3rd edition. New York: The
Benjamin/Cummings Publishing.
Carroll, R. L. 1964. The earliest reptiles. Zoological Journal
of the Linnean Society 45: 61-83.
Gaffney, E. S. 1980. Phylogenetic relationships of the major
groups of amniotes. In A. L. Panchen (ed.) The Terrestrial
Environment and the Origin of Land Vertebrates: 593-610.
London: Academic Press.
Gauthier, J. A. 1994. The diversification of the amniotes. In
D. R. Prothero and R. M. Schoch (ed.) Major Features
of Vertebrate Evolution: 129-159. Knoxville: The Paleontological
Society.
Gauthier J., R. Estes, & K. de Queiroz. 1988a. A phylogenetic
analysis of Lepidosauromorpha. In: R. Estes and G.
Pregill (eds.) Phylogenetic relationships of the lizard
families: 15-98. Stanford: Stanford University Press.
Gauthier, J., A. G. Kluge, & T. Rowe. 1988b. Amniote phylogeny
and the importance of fossils. Cladistics 4: 105-209.
Gauthier, J., A. G. Kluge, & T. Rowe. 1988c. The early evolution
of the Amniota. In M. J. Benton (ed.) The phylogeny
and classification of the tetrapods, Volume 1: amphibians,
reptiles, birds: 103-155. Oxford: Clarendon Press.
Goodman, M., M. Miyamoto, and J. Czelusniak. 1987. Pattern
and process in vertebrate phylogeny revealed by coevolution
of molecules and morphologies. In C. Patterson (ed.)
Molecules and Morphology in Evolution: Conflict or
Compromise?: 141-176. Cambridge: Cambridge University
Press.
Hedges, S. B. 1994. Molecular evidence for the origin of birds.
Proceedings of the National Academy of Sciences of
the United States of America 91:2621-2624.
Hedges, S. B., K. D. Moberg, and L. R. Maxson. 1990. Tetrapod
phylogeny inferred from 18S and 28S ribosomal RNA sequences
and a review of the evidence for amniote relationships.
Molecular Biology and Evolution 7:607-633.
Kumazawa, Y., and M. Nishida. 1995. Variations in mitochondrial
tRNA gene organization of reptiles as phylogenetic
markers. Molecular Biology and Evolution 12:759-772.
Laurin, M. & R. R. Reisz. 1995. A reevaluation of early amniote
phylogeny. Zoological Journal of the Linnean Society
113: 165-223.
Lee, M., S. Y. 1993. The origin of the turtle body plan: bridging
a famous morphological gap. Science 261: 1716-1720.
Lee, M. The turtle's long-lost relatives. Natural History, April
1994, 63-65.
Lee, M. S. Y. 1995. Historical burden in systematics and the
interrelationships of 'Parareptiles'. Biological Reviews
of the Cambridge Philosophical Society 70: 459-547.
Lee M. S. Y. 1996. Correlated progression and the origin of turtles.
Nature 379: 812-815.
Reisz, R. R., & M. Laurin. 1991. Owenetta and the origin of turtles.
Nature 349: 324-326.
Rieppel, O. 1994. Osteology of Simosaurus gaillardoti and the
relationships of stem-group sauropterygia. Fieldiana
Geology 1462: 1-85.
Rieppel O. 1995. Studies on skeleton formation in reptiles: implications
for turtle relationships. Zoology-Analysis of Complex
Systems 98: 298-308.
We thank Dr. David Maddison for many useful comments on this page,
and Ms. Patricia Lai for proof-reading the text.
Michel Laurin
E-mail: laurin@ccr.jussieu.fr.
URA CNRS 1137
"Evolution et adaptations des systèmes
ostéomusculaires"
Case 7077
Université Paris 7
Paris 75005
France.
Jacques A. Gauthier
E-mail: jgauthier@calacademy.org.
Department of Herpetology
California Academy of Sciences
Golden Gate Park
San Francisco, California 94118-9661
S. Blair Hedges
E-mail: SBH1@PSUVM.PSU.EDU.
Department of Biology and Institute of Molecular Evolutionary
Genetics
208 Mueller Laboratory
Pennsylvania State University
University Park, PA 16802
Correspondence regarding this page should be directed to Michel Laurin, at laurin@ccr.jussieu.fr.
Page copyright © 1996 Michel Laurin, Jacques A. Gauthier, and
S. Blair Hedges
Content changed June 7, 1996
Last saved 21 October 1998
Restored environment of Proganochelys (the oldest known turtle) during the Late Triassic in Germany. Besides Proganochelys, small hybodont sharks are shown. Painting by Frank Ippolito, copyright 1987 American Museum of Natural History. Reproduced with permission.