Trypanosoma
Julius Lukes- Trypanosoma congolense
- Trypanosoma cruzi
- Trypanosoma devei
- Trypanosoma minasense
- Trypanosoma rangeli
- Trypanosoma brucei
- Trypanosoma brucei equiperdum
- Trypanosoma brucei evansi
- Trypanosoma brucei brucei
- Trypanosoma brucei rhodesiense
- Trypanosoma brucei gambiense
- Trypanosoma carassii
- Trypanosoma rotatorium
- Trypanosoma theileri
- Trypanosoma melophagium
Note: this taxon list is still under construction. It does not yet contain all known Trypanosoma subgroups.
Introduction
The genus Trypanosoma is divided into several subgenera based on subtle morphological differences. However, the most important subdivision is into the groups Salivaria and Stercoraria, the latter involving species the development of which is terminated in the rear part of the digestive tract of the vector, here triatomine bugs. The transmission is therefore contaminative by excrements containing metacyclic trypomastigotes. In the vertebrate host, the stercorarian trypanosomes multiply as amastigotes or epimastigotes. Trypomastigote stages found in the peripheral blood do not divide and their sole function is to spread the infection into other organs and enable the transmission into the vector. T. theileri and T. melophagium are stercorarian species transmitted by tabanid and hipoboscid flies to cows and sheep, respectively.
However, by far the most important representative of the Stercoraria group is T. cruzi, the causative agent of Chagas disease in humans, which is able to infect at least 100 species of wild and domestic animals. There appear to be at least two groups of strains of T. cruzi that are genetically virtually isolated, although hybrids among them exist (Westenberger et al., 2005). The parasite is widespread in Central and South America and is responsible for enormous suffering and economic loss. The impact of Chagas disease is truly enormous, as estimated up to 20 million Latin Americans have different forms of the disease that usually has two main phases. The initial acute phase, usually accompanied by only mild symptoms (although exceptionally it can cause lethal endocarditis in children), is followed by an unusually long mostly asymptomatic phase (ranging from 10 to 40 years), during which progressive appearance of myocarditis and/or megaoesophagus and megacolon occur. Effective treatment of Chagas disease is lacking. The infection is diagnosed by the detection of trypanosmes in lymph nodes and peripheral blood (this is complicated by the existence of entirely non-pathogenic T. rangeli in the blood of humans and in triatomine bugs in South America), xenodiagnosis and recently, mostly by PCR (de Souza 2007).
The name of the other important group – Salivaria, is derived from “saliva”, since the development in the vector is terminated in the salivary glands. The transmission is thus inoculative, i.e. by the injection of infectious metacyclic trypanosomes during blood feeding of the vector. Trypanosomes then multiply in the host’s blood in the form of trypomastigotes. Salivarian trypanosomes apparently originated from the area where tse-tse flies (Glossina spp.) occur in Africa (the so-called tse-tse belt), and spread outside of this continent is accompanied by switching to other transmission routes, such as on the mouthparts of other blood-feeding insects or by the bite of a vampire bat.
The by far most important member of Salivarian trypanosomes (usually placed in the suborder Trypanozoon) is T. brucei, subdivided into the subspecies T. b. brucei, T. b. gambiense, T. b. rhodesiense, T. b. equiperdum, and T. b. evansi. Sometimes, these subspecies are recognized as separate species. Depending on their habitat, these trypanosomes are transmitted by a number of Glossina species, where they undergo a complex mode of development. T. brucei (brucei) is unable to infect humans, as it is lyzed by human serum that contains the so-called trypanosome lytic factor, which is a toxic type of the high density lipoprotein. This flagellate can be found in virtually every warm-blooded vertebrate species (usually antelopes, zebras, lions, beasts of burden, goats, pigs etc.), where it causes disease termed nagana (or N’gana) (Stuart et al., 2008).
T. (brucei) rhodesiense is, thanks to its resistance to human serum, capable of infecting humans. The inflicted disease is called (East-African) sleeping sickness and is usually lethal within about two months, if left untreated. While this acute form of sleeping sickness is encountered in the savannas of East Africa, being transmitted by savanna tse-tse flies, a somewhat less deadly form is confined to West Africa. There, the causative agent is T. (brucei) gambiense, transmitted by so-called river tset-tse flies. This chronic form, also called West-African sleeping sickness, causes death within two years of infection.
Sleeping sickness is diagnosed by observation of trypanosomes in the blood or punctuates of the lymph nodes. Serological tests, DNA hybridization and lately also PCR are being used as fast and sensitive diagnostic methods. Before progression into the central nervous system, the disease is treated with pentamidin; in the more advanced stage melarsoprol or eflornithin are prescribed (Brun and Balmer 2006).
Deadly diseases of camels, water buffaloes, horses and donkeys called dourine and surra are caused by two other subspecies of T. brucei, namely T. brucei equiperdum and T. brucei evansi. Both species were able to spread outside of Africa thanks to their capability of being transmitted via sexual intercourse or via the surface of the mouthparts of various blood-sucking insects, such as the tabanid flies. Recent research revealed that both species are in fact T. brucei strains that lost their mitochondrial (=kinetoplast) DNA either partially or fully, thus effectively becoming “petite” mutants of African trypanosomes (Lai et al., 2008).
References
Brun R. and O. Balmer. 2006. New development in human African trypanosomiasis. Curr. Opinion Infect. Dis. 19: 415-420.
De Souza W. 2007. Chagas disease: facts and reality. Microbes Infect. 9: 544-545.
Lai D.H., H. Hashimi, Z. Lun, F.J. Ayala and J. Lukeš. 2008. Adaptation of Trypanosoma brucei to gradual loss of kinetoplast DNA: T. equiperdum and T. evansi are petite mutants of T. brucei. Proc. Natl. Acad. Sci. USA 105: 1999-2004.
Stuart K., R. Brun, S. Croft, A. Fairlamb, R.E. Gurtler, J. McKerrow, S. Reed and Tarleton, R. 2008. Kinetoplastids: related protozoan pathogens, different diseases. J. Clin. Invest. 118: 1301-1310.
Westenberger S.J., C. Barnabe, D.A. Camnpbell and N.R. Sturm. 2005. Two hybridization events define the population structure of Trypanosoma cruzi. Genetics 171: 527-543.
Title Illustrations

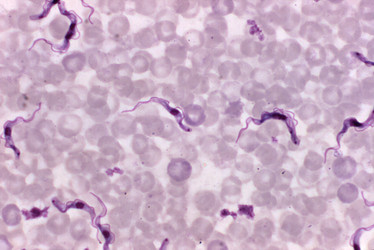
| Scientific Name | Trypanosoma brucei |
|---|---|
| Creator | CDC/ Dr. Mae Melvin |
| Source Collection | Public Health Image Library (Centers for Disease Control) |
About This Page
This page is being developed as part of the Tree of Life Web Project Protist Diversity Workshop, co-sponsored by the Canadian Institute for Advanced Research (CIFAR) program in Integrated Microbial Biodiversity and the Tula Foundation.

University of South Bohemia in Ceske Budejovice, Czech Republic
Correspondence regarding this page should be directed to Julius Lukes at
Page copyright © 2009
All Rights Reserved.
- First online 02 January 2009
- Content changed 02 January 2009
Citing this page:
Lukes, Julius. 2009. Trypanosoma . Version 02 January 2009 (under construction). http://tolweb.org/Trypanosoma/98034/2009.01.02 in The Tree of Life Web Project, http://tolweb.org/







 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site