Aleocharinae
James S. Ashe (1947-2005)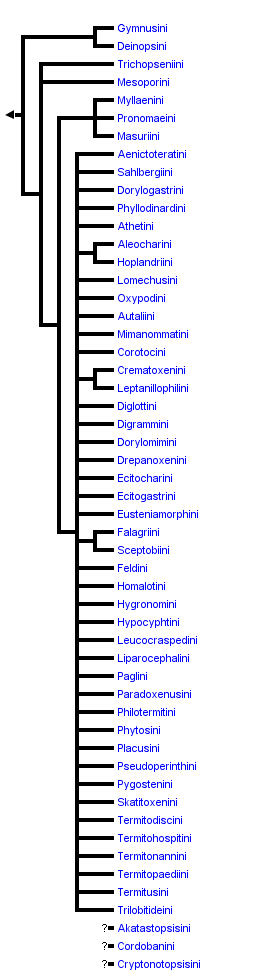


This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxThree tribes listed in Newton and Thayer (1992) are not included in this phylogeny: Actocharini Bernhauer and Scheerpeltz 1911; Diestotini Mulsant and Rey 1871; Heterotaxini Fenyes 1921. Currently there is no phylogenetic basis for recognizing these tribes, Consequently, I follow Newton (unpublished manuscript) in including the Actocharini and Diestotini in the Homalotini, and the Heterotaxini in the Hygronomini.
Introduction
The Aleocharinae is one of the largest lineages, and taxonomically the most difficult lineage, of staphylinid beetles. It includes 52 tribes, over 1,000 described and probably valid genera and over 12,000 described species. The great described diversity of these beetles only hints at the true diversity of aleocharines, with many thousands of species, and numerous higher taxa, remaining to be described from throughout the world, especially in tropical regions.
Aleocharines are mostly small to minute beetles. A few are as large as 10 millimeters in length, but the vast majority are 3-5 millimeters in length, and a few are 1 millimeter or less in length, making them among the smallest of beetles. The body color of most aleocharines is various shades of light to dark brown, reddish-brown or black, but some are light to dark yellowish, and some have bodies with striking, contrasting colors of yellow or red and black. Aleocharines are distributed throughout the world in virtually all terrestrial habitats, where they are among the most abundant and diverse of the microcoleoptera in many microhabitats.
Characteristics
Hammond (1975) and Ashe (1994) showed that the extremely large, complex and multiarticulated lateral lobes (parameres) of the aedeagus were unique to the staphylinid lineage treated here as the Aleocharinae and hypothesized that such parameres were a synapomorphy indicating the monophyly of this group.

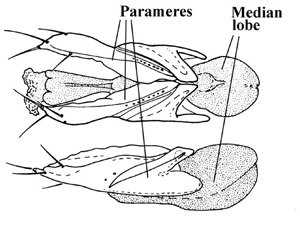
Dorsal and lateral aspects of the aedeagus of a relatively basal aleocharine, Anacyptus testaceus (tribe Mesoporini) showing the large lateral lobes (parameres) and the median lobe.
Ashe and Newton (1993) confirmed the monophyly of the Aleocharinae based on the larval characters of: 6-8 pores in medial transverse row of epipharynx; mandible with a noticably enlarged molar lobe; and, 3 or fewer stemmata.
Many taxa also have the antennae inserted between and near the anterior margins of the eyes, but many aleocharines lack this latter characteristic even though it is often used in keys for identification.
Habits and Habitats
Aleocharines represent one of the great monophyletic radiations in the history of life. This radiation is characterized by dramatic habitat, microecological and behavioral specialization in various lineages. While many aleocharines are dominant generalist predators in leaf litter and soil communities, others have very specialized habits and habitats. In terms of the numbers of lineages and species, aleocharines are by far the most successful group of inquilines in the nests of social insects, especially in ant and termite nests (Seevers 1957, 1965, and others). Several lineages are associated with mushrooms and fungi, and at least one of these has become exclusively mycophagous (Ashe 1984, 1986, 1992). Aleocharines are one of the very few lineages of insects to invade and diversify in the intertidal zones of seashores (Moore and Legner 1976, Ahn 1996, Ahn and Ashe 1992, 1995, 1996a,b). Some aleocharines are found exclusively under bark of dead trees and logs. Members of the genus Aleochara are "parasites" of Diptera puparia (Peschke and Fuldner 1977, Klimaszewski 1984). Recently, some aleocharines (Amazoncharis spp.) have been found to be major pollinators of some palm trees in South America (Bernal and Ervik 1996). Adults and larvae of the genus Tachiona are found exclusively in the web-covered burrows of hepialid moth caterpillars (Ashe 1990), and larvae and adults of Charoxus are found inside the receptacles of ripe figs (Kistner 1981, Ashe unpubl. data)]. Many other examples of unusual specialization among aleocharines could be cited.
Identification Guides
The seemingly endless diversity, the small size of most adults, and the virtual lack of illustrated keys and descriptions of aleocharines for most geographical regions make the Aleocharinae one of the most taxonomically difficult groups of beetles. For example, Casey (1906, 1911), who described most North American aleocharines, did not provide keys to most taxa, and recent comprehensive identification guides (Arnett 1968, Moore and Legner 1974, 1979) did not provide keys to aleocharine genera. Consequently, it is virtually impossible for a specialist in the Staphylinidae to identify the vast majority of aleocharines from most geographic regions even to genus, much less to species, using the available literature. That said, there are a few regions for which the aleocharine fauna is relatively well known, and for which adequate identification guides have been published.
Europe is the only region for which aleocharines are well known and for which one can identify most aleocharines using available guides. These include guides to the aleocharine fauna of middle Europe (Lohse 1974), Denmark (Hansen 1954), and England (Joy 1976).
Seevers (1978) provided keys, and descriptions of many genera, for the aleocharine genera of America north of Mexico, but these keys are very diffucult to use (even for a specialist in the Aleocharinae) and reflect the fact that Seevers' work was not yet complete at the time of his death. Downie and Arnett (1996) provided keys to the aleocharines of the Northeastern United States, but these keys are also very difficult to use and lack illustrations of characters. In addition, Lohse, Klimaszewski and Smetana (1990) provided keys, illustrations of diagnostic features and descriptions of the aleocharines of arctic Canada and Alaska.
Cameron (1939a,b) provided keys and descriptions of the genera and species of India; these keys provide a basic reference (though incomplete) for identifying genera of aleocharines throughout the Oriental faunal region. Cameron (1921) also provided keys to the aleocharine (and other staphylinid) genera of Singapore; these are also useful for identification of aleocharine genera of the Oriental region though they are not illustrated and many taxa are not included.
A few identification guides are available as WWW resources: J. S. Ashe provided a key to the described Mexican aleocharine genera with many taxa illustrated by photographs, and an overview of the known diversity and distribution of Mexican aleocharines; K.-J. Ahn provided a completely illustrated key to the intertidal aleocharine genera of North America and a complete checklist of the intertidal species. Ashe and his associates also provided an image database that includes illustrations (photographs, line drawings and scanning electron micrographs) of 330 genera. These resources are accessible from the J. S. Ashe PEET Project Page.
In addition to these more general guides, several tribes or subtribes have received recent revision; these revisions provide keys, illustrations of diagnostic characters, and descriptions for identification of included genera. These include: subtribe Gyrophaenina (Ashe 1984), subtribe Bolitocharina (Ashe 1992), Sceptobiini (Danoff-Burg 1994), Liparocephalini (Ahn and Ashe 1996a), Falagriini of North America (Hoebeke 1985, Ahn and Ashe 1995), Gymnusini-Deinopsini (Klimaszewski 1979), North American Aleocharini (Aleochara) (Klimaszewski 1984) and Myllaenini (Myllaena) (Klimaszewski 1982b), Aenictoteratini (Kistner 1993), Leptanillophilini (Jacobson and Kistner 1991), Crematoxenini (Jacobson and Kistner 1992), Corotocini (Jacobson, Kistner and Pasteels 1986), Dorylogastrini (Kistner 1993), Dorylomimini (Kistner 1993), Ecitocharini (Kistner and Jacobson 1990), Feldini (Kistner 1972), Coptotermoeciina (Kistner and Pasteels 1970a), Mimanommatini (Kistner 1993), Pseudoperinthini (Kistner and Pasteels 1970b), Pygostenini (Jacobson and Kistner 1975), Sahlbergiini (Kistner 1993), Trichopseniini (Pasteels and Kistner 1971).
Finally, Seevers (1957, 1965) provided guides to the Aleocharinae (and other staphylinids) associated with termites (1957) and army ants (1965). These include habitus illustrations of representatives of many genera, but these works have few keys and are difficult for the non-specialist to use.
Phylogenetic Position of Aleocharinae Within the Staphylinidae
Aleocharines are members of a group of staphylinid subfamilies called the "Tachyporine Group" (Lawrence and Newton 1982). The Tachyporine Group comprises 6 subfamilies: 1) Phloeocharinae (seven genera, Holarctic and Australian); 2) Olisthaerinae (one genus, Holarctic); 3) Tachyporinae (34 genera in 7 tribes, Worldwide); 4) Habrocerinae (two genera, Holarctic, Brazil and Chile); 5) Trichophyinae (one genus; Holarctic and Oriental); and 6) Aleocharinae (over 1,000 genera in 52 tribes, Worldwide) (Ashe and Newton 1993). Phylogeny of lineages within the Tachyporine Group has been obscure though it is presumed to be a monophyletic lineage. Recently, Ashe and Newton (1993) provided and discussed a phylogeny of major genera and subfamilies based on larval characteristics. However, larval characters did not provide support for a monophyletic Tachyporine Group, the subfamily Tachyporinae was not supported as monophyletic - various tachyporine genera arise in five different lineages - and larval characters did not provide robust resolution of the phylogeny of all taxa included in the analysis. However, this analysis provides strong support that the Aleocharinae are monophyletic based on larval characters. Consequently, both Hammond (1975) and Ashe and Newton (1993) have shown that the Aleocharinae are monophyletic based on aedeagal and larval structures.
In contrast to these conclusions, Naomi (1985) divided those staphylinids treated by Hammond (1975) and Ashe and Newton (1993) as the subfamily Aleocharinae into 7 subfamilies which he called the "Aleocharine Subgroup". He limited the subfamily Aleocharinae more-or-less to those taxa treated below as the "higher" Aleocharinae (some of the aleocharine subgroup "subfamilies" that Naomi recognized also have tergal glands characteristic of the "higher" Aleocharinae). However, Newton and Thayer (1988) showed that Naomi's analysis is flawed, and that, when Naomi's characters are reinterpreted and reanalyzed, they do not support his phylogenetic conclusions.
Discussion of Phylogenetic Relationships
In spite of the abundance and probable importance of Aleocharinae in many habitats and their amazing diversity, very little phylogenetic work has been attempted on the group. There have been no comprehensive studies, and the relatively few past studies of phylogeny in this large group have been addressed by examining phylogenetic structure in higher taxon subunits. This has been the approach taken by my students and me for the subtribe Gyrophaenina (Ashe 1984, 1986), subtribe Bolitocharina (Ashe 1992), Sceptobiini (Danoff-Burg 1994) and Liparocephalini (Ahn and Ashe 1996a), and also for the Falagriini (Hoebeke 1985, Ahn and Ashe 1995) and Gymnusini-Deinopsini (Klimaszewski 1979) and a number of groups of termitophiles and myrmecophiles (Jacobson and Kistner 1991, 1992 Jacobson et al 1986, Kistner and Jacobson 1990). In addition Steidle and Dettner (1993) provided a very preliminary phylogeny of a few selected groups based on structure of the abdominal tergal gland and the chemicals secreted.
Consequently, the classification of this unusually diverse array of insects is extremely artificial. The basic features of the classification were established by Erichson (1837, 1840), based on the number of tarsal elements, number of segments in the maxillary and labial palpi and the highly autapomorphic features shared by members of some lineages. This system produces a classification that has little phylogenetic basis, and indeed, some higher taxa are known to be non-monophyletic (for example, the large tribe Oxypodini is based on the presence of 5-5-5 tarsal segmentation, a character that is well known to be plesiomorphic.) This classification has been acknowledged to be artificial and inadequate by a number of aleocharine workers (Fenyes 1918-21, Seevers 1978 and many others); however, the large size and taxonomic difficulty of the group has always prevented comprehensive phylogenetic study.
The phylogeny of tribes provided above is a synthesis compiled from phylogenetic statements, comments and phylogenies of various higher taxa provided by a number of individual authors and in numerous papers. The evidence for each branch indicated on this tree is provided below. However, because a comprehensive higher level phylogeny has not been done, all branches of the phylogeny provided here should be considered highly tentative and in need of further study. It is also important to remember that the monophyly of most tribes has not been rigorously tested. The support for monophyly (or lack thereof) for each tribal level taxon will be discussed in the Tree-of-Life page for that taxon.
The Aleocharinae can be divided into 2 informal groups: the "basal" Aleocharinae, and the "higher" Aleocharinae. The "basal" aleocharines are made up of the Gymnusini (2 genera), Deinopsini (3 genera), Mesoporini (including Paraconosoma, 6-8 genera) and Trichopseniini (15 genera). Members of these tribes are considered "basal" because they lack the highly derived (and unique) tergal gland and associated structures at the base of tergum VII of adults and at the apex of tergum VIII of larvae, as well as a number of derived features in the aedeagus, that characterize the much more diverse "higher" Aleocharinae (Hammond 1975, Ashe and Newton 1993, Ashe 1994). The "basal" aleocharines, characterized by a shared plesiomorphic condition, are not a monophyletic group (they are paraphyletic in relation to the "higher" Aleocharinae). However, the "higher" aleocharines (which include all other aleocharine taxa) are characterized by a complex and unique synapomorphy (presence of the tergal gland in both larvae and adults) and almost certainly form a monophyletic lineage. Steidle and Dettner (1993) have provided a detailed discussion of the morphology of tergal glands of adult aleocharines and a discussion of the chemical secretions of these glands among selected groups of aleocharines.
Hammond (1975) first proposed a sister group relationship between the tribes Gymnusini and Deinopsini based on shared derived characters in the mouthparts of adults and the presence of pectinate edges of the apical margins of abdominal segments III-V of adults (a feature unique in the Staphylinidae), and Klimaszewski (1979) confirmed this relationship. Ashe and Newton (1993) also confirmed a sister group relationship between the Gymnusini and Deinopsini based on larval characters. Derived larval characters shared between these two lineages include: preapical tooth of mandible displaced dorsally; molar area of mandibles with spiniform processes; mala of maxilla with long, spiniform, falcate apex and large basal spine well separated from small medial spines; and, labial and maxillary palpi with apical pseudosegment.
The position of the tribe Myllaenini has been problematic. Seevers (1978) and Klimaszewski (1982b) hypothesized that the Myllaenini is a member of the "basal" Aleocharinae and is related to the Gymnusini and Deinopsini. They based this hypothesis primarily on similarity in body form (all are teardrop-shaped, with strongly deflexed head), stylate mouthparts, and the fact that all are found in riparian habitats. However, Hammond (1975) and Ashe and Newton (1993) presented evidence that they are actually members of the "higher" Aleocharinae. Presence of a tergal gland in both larvae and adults of Myllaenini supports the latter conclusion. Steidle and Dettner (1993) suggested that the Myllaenini were the most basal group of the higher aleocharines that they examined based on the very small opening of the tergal gland of Myllaena (the only taxon of the Myllaenini sampled).
Seevers (1978) proposed that the Pronomaeini, Masuriini and Dimonomerini were related to the Myllaenini based on similarity in mouthpart structure.
Seevers (1978) noted that the members of the tribes Aleocharini and Hoplandriini shared presence of a pseudosegment on the maxillary palpi and an unusual reticulated array of sclerotized supports in the velum of the parameres. Based on these characters he proposed that they form a monophyletic group.
Jacobson and Kistner (1991, p. 5) point out that the closest relatives of the Leptanillophilini are the Crematoxenini. However, Jacobson and Kistner (1992) stated that the Crematoxenini are related to the Ecitopora and Dinocoryna groups of the Zyrini (= Lomeschusini). In addition, Kistner (1968) proposed that the tribe Termitopaedini was closely related to the "Myrmedoniini" (= Lomeschusini) with which it shares the 4-5-5 tarsal formula and generalized head structure. However, I have not reflected this conclusion in the phylogeny above because the phylogenetic information in these characters is highly questionable.
Seevers (1965) suggested a phylogenetic relationship between the tribes Deremini and Athetini (Scheerpeltz 1934 placed the Deremini in the "Myrmedoniini" near Falagria), but Kistner and Jacobson (1979) pointed out that the Deremini lack the "athetine bridge" that characterizes the median lobe of the Athetini. Seevers (1965) also included the genus Dorylophila in the genus Derema, which led Newton and Thayer (1992) to point out that the tribe Deremini is a junior synonym of the tribe Dorylophilini. Therefore, I have not included the Deremini, nor have I reflected the possibility of a relationship between the Deremini and the Athetini, in the phylogeny above.
More recently, Kistner (1993) placed the Dorylophilini as a subtribe within the tribe Mimanommatini, and raised four former mimanommatine subtribes to tribal status: the Dorylogastrini, Dorylomimini, Aenictoteratini and Sahlbergiini.
Seevers (1978) first proposed that the tribes Falagriini and Sceptobiini together formed a monophyletic group based on shared presence of a divided velum of the paramere. Danoff-Burg (1994) and Ahn and Ashe (1995) provided additional evidence for the monophyly of the Falagriini + Sceptobiini.
Steidle and Dettner (1993) suggested that the tribes Oxypodini, Athetini, Lomechusini (they called it the "Myrmedoniini"), and Aleocharini form a monophyletic group based on shared presence of topically extremely effective hydrocarbons, aldehydes and 1,4-benzoquinone or 1,4-hydroquinone in the secretions from the tergal gland among examined members of these groups. In addition, the Aleocharini, Lomeschusini and Athetini share the presence of a large gland reservoir and should be separated from the Oxypodini which have a small reservoir, small number of gland cells and prevalence of acid esters in the secretions. I have not reflected this hypothesis in the phylogeny included here because they examined so few representatives of each group, and they did not examine representatives of many other taxa that could be related to one or the other of these higher taxa. It could be misleading to include the hypothesis that these 4 tribes form a monophyletic group in comparison to other tribes in the phylogeny presented here.
References
Ahn, K.-J. 1996. A review of Diaulota Casey (Coleoptera: Staphylinidae: Aleocharinae) with description of new species and known larvae. Coleopts Bull. 50(3): 270-290.
Ahn, K.-J. and J. S. Ashe. 1992. Revision of the intertidal aleocharine genus Pontomalota Casey (Coleoptera: Staphylinidae) with a discussion of phylogenetic relationships. Ent. Scand. 23: 347-359.
Ahn, K.-J. and J. S. Ashe. 1995. Systematic position of the intertidal genus Bryobiota Casey and a revised phylogeny of the falagriine genera of America north of Mexico (Coleoptera: Staphylinidae: Aleocharinae). Ann. Entomol. Soc. Amer. 88(2): 143-154.
Ahn, K-J. and J. S. Ashe. 1996a. Phylogeny of the intertidal aleocharine tribe Liparocephalini (Coleoptera: Staphylinidae). Systematic Entomology 21: 99-114.
Ahn, K-J. and J. S. Ashe. 1996b. A revision of Rothium Moore and Legner (Coleoptera: Staphylinidae: Aleocharinae) with a discussion of phylogenetic relationships. Jour. Kansas Entomol. Soc. 69(3): 234-256.
Arnett, R. H. Jr. 1968. The beetles of the United States (A manual for identification). Amer. Entomol. Inst., Ann Arbor.
Ashe, J. S. 1984. Generic revision of the subtribe Gyrophaenina (Coleoptera: Staphylinidae: Aleocharinae) with a review of described subgenera and major features of evolution. Quest. Entomol. 20: 129-349.
Ashe, J. S. 1986. Structural features and phylogenetic relationships among larvae of genera of gyrophaenine staphylinids Coleoptera: Staphylinidae: Aleocharinae). Fieldiana: Zoology, n. s. 30: 1-60.
Ashe, J. S. 1990. New species, phylogeny and natural history of Tachiona Sharp 1883 (Coleoptera: Staphylinidae: Aleocharinae). Trop. Zool. 3: 225-235.
Ashe, J. S. 1992. Phylogeny and revision of genera of the subtribe Bolitocharina (Coleoptera: Staphylinidae: Aleocharinae). Univ. Kansas Sci. Bull. 54: 335-406.
Ashe, J. S. 1994. Evolution of aedeagal parameres of aleocharine staphylinids (Coleoptera: Staphylinidae: Aleocharinae). Can. Entomol. 126: 475-491.
Ashe, J. S. and A. F. Newton, Jr. 1993. Larvae of Trichophya and phylogeny of the Tachyporine Group of subfamilies (Coleoptera: Staphylinidae) with a review, new species and characterization of the Trichophyinae. Syst. Entomol. 18: 267-286.
Bernal, R. and F. Ervik. 1996. Floral biology and pollination of the dioecious palm Phytelephas seemannii in Colombia: An adaptation to staphylinid beetles. Biotropica 28(4b): 682-686.
Cameron, M. 1921. New species of Staphylinidae from Singapore, Part IV (Conclusion). Transactions of the Entomological Society of London (1921): 347-413.
Cameron, M. 1939a. The fauna of British India, including Ceylon and Burma. Coleoptera, Staphylinidae, Vol.IV, part I. W. Junk, the Hague. pp. 1-410.
Cameron, M. 1939b. The fauna of British India, including Ceylon and Burma. Coleoptera, Staphylinidae, Vol.IV, part II. W. Junk, the Hague. pp. 411-691, 3 plates, 1 map.
Casey, T. L. 1906. Observations on the staphylinid groups Aleocharinae and Xantholinini. Trans. Acad. Sci. Saint Louis 16: 125-434.
Casey, T. L. 1911. New American species of Aleocharinae and Myllaeninae. Mem. Coleopt. 2: 1-259.
Danoff-Burg, J. A. 1994. Evolving under myrmecophily: a cladistic revision of the symphilic beetle tribe Sceptobiini (Coleoptera: Staphylinidae: Aleocharinae). Syst. Entomol. 19: 25- 45.
Downie, N. M. and R. H. Arnett. 1996. The beetles of Northeastern North America. Vol. I. Sandhill Crane Press, Gainsville, Florida. 880 pp.
Erichson, W. F. 1837. Die Käfer der Mark Brandenburg. Vol. 1, Abt. 1. F. H. Morin, Berlin. viii + 384 pp.
Erichson, W. F. 1840. Genera et species staphylinorum insectorum coleopterorum familiae. Part 2. F. H. Morin, Berlin. pp. 401-954.
Fenyes, A. 1918-21. SubFam. Aleocharinae. Genera Insectorum 173. Pasadena.
Hammond, P. M. 1975. The phylogeny of a remarkable new genus and species of gymnusine staphylinid (Coleoptera) from the Auckland Islands. Jour. Entomol. (B) 44: 153-173.
Hansen, V. 1954. Dannmarks Fauna. Bd. 59. Biller XVII, Rovbiller 3 (Aleocharinae). Copenhagen. 499 pp., 361 figs.
Hoebeke, E. R. 1985. A revision of the rove beetle tribe Falagriini of America north of Mexico. Jour. New York Entomol. Soc. 93: 913-1018.
Jacobson, H. R. and D. H. Kistner. 1975. A manual for identification of the Pygostenini. Sociobiology 1(3): 201-335.
Jacobson, H. R. and D. H. Kistner. 1991. Cladistic study, taxonomic restructuring, and revision of a myrmecophilous tribe Leptanillophilini with comments on its evolution and host relationships (Coleoptera: Staphylinidae; Hymenoptera: Formicidae). Sociobiology 18: 1- 150.
Jacobson, H. R. and D. H. Kistner. 1992. Cladistic study, taxonomic restructuring, and revision of the myrmecophilous tribe Crematoxenini with comments on its evolution and host relationships (Coleoptera: Staphylinidae; Hymenoptera Formicidae). Sociobiology 20: 91- 201.
Jacobson, H. R., D. H. Kistner, and J. M. Pasteels. 1986. Generic revision, phylogenetic classification, and phylogeny of the termitophilous tribe Corotocini (Coleoptera: Staphylinidae) Sociobiology 12: 1-245.
Joy, N. H. 1976. A practical handbook of British Beetles. Vol. 1 (text), Vol. 2 (plates). E. B. Classey Ltd. Vol. 1 (1-149), Vol. 2 (1-43).
Kistner, D. H. 1968. A taxonomic revision of the termitophilous tribe Termitopaedini, (Coleoptera: Staphylinidae) with notes on behavior, systematics, and post-imaginal growth. Misc. Pubs. Entomol. Soc. Amer. 6(3): 141-196.
Kistner, D. H. 1972. A revision of the termitophilous tribe Feldini (Coleoptera Staphylinidae) with a numerical analysis of the relationships of the species and gnera. Contributions of the American Entomological Institute 8(4): 1-36.
Kistner, D. H. 1981. The reclassification of the genus Charoxus Sharp with the description of new species (Coleoptera: Staphylinidae). Journal of the Kansas Entomological Society 54(3): 587-598.
Kistner, D. H. 1993. Cladistic analysis, taxonomic restructuring and revision of the Old World genera formerly classified as Dorylomimini with comments on their evolution and behavior (Coleoptera: Staphylinidae). Sociobiology 22(2): 151-383.
Kistner, D. H. and H. R. Jacobson. 1979. Revision of the myrmecophilous tribe Deremini, III. The remainder of the genera with notes on behavior, ultastructure, glands and phylogeny (Coleoptera: Staphylinidae). Sociobiology 3(3): 143-393.
Kistner, D. H. and H. R. Jacobson. 1990. Cladistic analysis and taxonomic revision of the ecitophilous tribe Ecitocharini with studies of their behavior and evolution (Coleoptera, Staphylinidae, Aleocharinae). Sociobiology 17: 333-480.
Kistner, D. H. and J. M. Pasteels. 1970a. Taxonomic revision of the termitophilus subtribe Coptotermoeciina (Coleoptera: Staphylinidae) with a discussion of some integumentary glands and a numerical analysis of their relationships. Pacific Insects 12(1): 85-115.
Kistner, D. H. and J. M. Pasteels. 1970b. Revision of the termitophilous tribe Pseudoperinthini (Coleoptera: Staphylinidae) with a discussion of some integumentary glands and the relationships of termitophiles with their hosts. Pacific Insects 12(1): 67-84.
Klimaszewski, J. 1979. A revision of the Gymnusini and Deinopsini of the World (Coleoptera: Staphylinidae: Aleocharinae). Agriculture Canada Monograph 25: 1-169.
Klimaszewski, J. 1982a. Studies of Myllaenini (Coleoptera: Staphylinidae: Aleocharinae), 1. Systematics, phylogeny and zoogeography of Nearctic Myllaena Erichson. Can. Entomol. 114: 181-240.
Klimaszewski, J. 1982b. A redefinition of the Myllaenini Ganglbauer and redescriptions of Camacopalpus Motschulsky and Polypea Fauvel (Coleoptera: Staphylinidae). Can. Entomol. 114: 411-429.
Klimaszewski, J. 1984. A revision of the genus Aleochara Gravenhorst of America north of Mexico (Coleoptera: Staphylinidae: Aleocharinae). Mem. Entomol. Soc. Can. 129: 211 pp.
Lawrence, J. F. and A. F. Newton, Jr. 1982. Evolution and Classification of beetles. Ann. Rev. Ecol. Syst. 13: 261-290.
Lohse, G. A. 1974. Die Kaefer Mitteleuropas. Bd. 5. Staphylinidae II (Hypocyphtinae und Aleocharinae). Pselaphidae. Goeke & Evers., Krefeld.
Lohse, G. A., J. Klimaszewski and A. Smetana. 1990. Revision of the Arctic Aleocharinae (Coleoptera: Staphylinidae). Coleopts Bull. 44: 121-202.
Moore, I. and E. F. Legner. 1974. Keys to the genera of Staphylinidae of America north of Mexico exclusive of the Aleocharinae (Coleoptera: Staphylinidae). Hilgardia 42(16): 548- 563.
Moore, I and E. F. Legner. 1976. Intertidal rove beetles (Coleoptera: Staphylinidae). pp. 521- 551. In, Cheng, L. (ed.). Marine Insects. North-Holland Publ. Co., Amsterdam.
Moore, I. and E. F. Legner. 1979. An illustrated guide to the genera of the Staphylinidae of America north of Mexico exclusive of the Aleocharinae. Univ. of Calif., Berkeley, No. 4093. 1-332.
Naomi, S.-I. 1985. The phylogeny and higher classification of the Staphylinidae and their allied groups. Esakia 29: 1-27.
Newton, A. F., Jr. Unpublished Manuscript. Systematic list of generic names of the Staphylinidae. Field Museum of Natural History, 1992. 61 pp.
Newton, A. F., Jr. and M. K. Thayer. 1988. A critique on Naomi's phylogeny and higher classification of Staphylinidae and allies (Coleoptera). Entomol. Gener. 14(1): 63-72.
Newton, A. F., Jr. and M. K. Thayer. 1992. Current classification and Family-Group names in Staphyliniformia (Coleoptera). Fieldiana:Zoology, new series No. 67: 1-92.
Pasteels, J. M. and D. H. Kistner. 1971. Revision of the termitophilous subfamily Trichopseniinae (Coleoptera: Staphylinidae). II. The remainder of the genera with a representational study of the gland systems and a discussion of their relationships. Misc. Publications of the Entomological Society of America 7(4): 351-399.
Peschke, K. and D. Fuldner. 1977. Uebersicht und neue Untersuchungen zur Lebensweise der parasitoiden Aleocharinae (Coleoptera: Staphylinidae). Zool. Jb. Syst. 104: 242-262.
Scheerpeltz, O. 1934. Staphylinidae VIII: Supplementum II, pp. 1501-1881. In, Schenkling, S., ed., Coleopterorum Catalogus, Pars 129. W. Junk, Berlin.
Seevers, C. H. 1957. A monograph on the termitophilous Staphylinidae (Coleoptera). Fieldiana: Zoology 40: 1-334.
Seevers, C. H. 1965. The systematics, evolution and zoogeography of staphylinid beetles associated with army ants (Coleoptera: Staphylinidae). Fieldiana: Zoology 47(2): 1-351.
Seevers, C. H. 1978. A generic and tribal revision of the North American Aleocharinae (Coleoptera: Staphylinidae). Fieldiana: Zoology 71: vi - 275.
Steidle, J. L. M. and K. Dettner. 1993. Chemistry and morphology of the tergal gland of freeliving Aleocharinae (Coleoptera: Staphylinidae). Syst. Entomol. 18: 149-168.
Title Illustrations

| Scientific Name | Cordalia cordicollis |
|---|---|
| Location | Europe |
| Size | length 2.5 mm |
| Copyright |
© 1998 James S. Ashe (1947-2005)

|
| Scientific Name | Tachiona deplanata |
|---|---|
| Location | Mexico |
| Size | length 6.1 mm |
| Copyright |
© 1998 James S. Ashe (1947-2005)

|
| Scientific Name | Xenodusa cava |
|---|---|
| Location | North America (Kansas) |
| Size | length 6.8 mm |
| Copyright |
© 1998 James S. Ashe (1947-2005)

|
| Scientific Name | Labidoglobus nevermanni |
|---|---|
| Location | Costa Rica |
| Size | length 1.5 mm |
| Copyright |
© 1998 James S. Ashe (1947-2005)

|
About This Page
Development of this page made possible by National Science Foundation PEET grant DEB 95-21755 to James S. Ashe. All images on this page copyright © 1997 James S. Ashe.
James S. Ashe (1947-2005)

University of Kansas, Lawrence, Kansas, USA
Correspondence regarding this page should be directed to James S. Ashe (1947-2005) at
Page copyright © 1998 James S. Ashe (1947-2005)
All Rights Reserved.
- First online 29 August 1997
- Content changed 25 April 2007
Citing this page:
Ashe (1947-2005), James S. 2007. Aleocharinae. Version 25 April 2007. http://tolweb.org/Aleocharinae/9777/2007.04.25 in The Tree of Life Web Project, http://tolweb.org/













 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site