Anthozoa
Sea Anemones, Corals, Sea Pens
Daphne G. Fautin and Sandra L. Romano- Alcyonaria (soft corals, sea pens)
- Zoantharia (sea anemones, stony corals, black corals)
Introduction
Anthozoans are exclusively marine, polypoid cnidarians. They include the familiar sea anemones, and other anemone-like groups with skeletons (such as the "stony" scleractinian corals) and without skeletons (such as tube anemones), as well as sea pens, sea fans, blue coral, and black coral. Anthozoans occur from the intertidal zone to the depths of the trenches (to 6000 m). In excess of 6000 species currently exist (Hyman 1940), comprising about two-thirds of extant cnidarian species (Dunn 1982); some anthozoans, such as the scleractinian corals, have a rich fossil history (Wells and Hill 1956).
An anthozoan polyp, like polyps in other cnidarian classes, consists of a tubular body terminating in a mouth surrounded by a ring of tentacles. The tentacles of an anthozoan polyp, unlike those of some other classes, are hollow, so the coelenteric space extends into them. The mesoglea between the ectoderm and the endoderm is relatively thick and cellular in many anthozoans compared to that of polyps in the other classes. The skeletal elements of some taxa are internal, lying in the thick mesogleal layer, whereas other anthozoans have skeletons lying entirely outside the living tissues (Fautin and Mariscal 1991).
Most anthozoan orders contain exclusively colonial species. An anthozoan colony consists of polyps connected by living tissue, the coenenchyme. The members of a colony are derived by vegetative (asexual) propagation from an original founder polyp that was the product of sexual reproduction, possibly with rare exceptions. At least some individuals of clonal species, like those of colonial species, propagate vegetatively by means such as fission, budding, or laceration, but, unlike in colonial species, the progeny do not remain attached to one another. Anthozoans of other species are incapable of asexual reproduction (e.g. Shick 1991).
Characteristics
Anthozoans are characterized by two anatomically related structures, the actinopharynx and the mesenteries, which are unique among cnidarian polyps (Figure 1). The actinopharynx, or stomodeum, is a tubular gullet extending from the mouth some distance into the coelenteron. The actinopharynx of most species contains at least one histologically specialized, flagellated longitudinal channel called a siphonoglyph that drives water into the coelenteron. In most sea anemones and corals, two siphonoglyphs are situated diametrically opposite one another in the actinopharynx. Siphonoglyphs and their associated structures impart a bilateral or biradial symmetry to the polyp (e.g. Shick 1991).

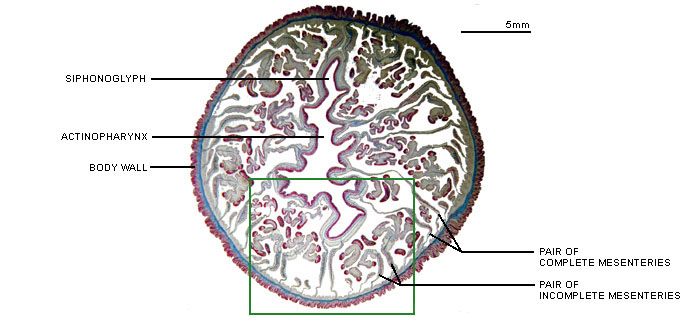

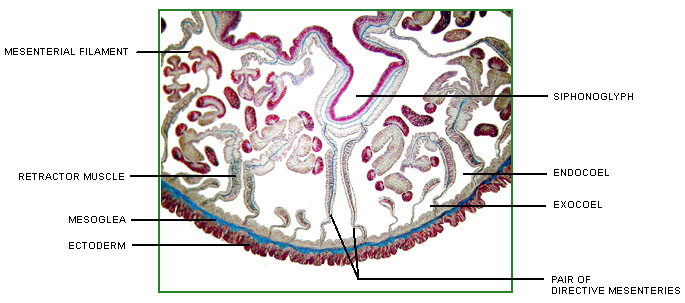
Figure 1. Cross-section of an anthozoan, the sea anemone Megalactis sp., at the level of the actinopharynx.
Image copyright © 2000 Adorian Ardelean.
Longitudinal sheets of tissue termed mesenteries extend radially from the body wall; some ("complete" or "perfect" ones) reach all the way to the actinopharynx. The mesenteries that attach to the siphonoglyphs may be anatomically specialized. Longitudinal retractor muscles of the mesenteries enable the polyp to retract. Along the free edge of each mesentery may be filaments provided with cilia, gland cells, and cnidae. The cilia probably circulate fluid that otherwise might stagnate in the compartments defined by the mesenteries; the cnidae and gland cells function in digestion (Fautin and Mariscal 1991). The term "septum," which is sometimes used as a synonym for mesentery, properly refers to the calcified radial partition of the scleractinian skeleton that lies between a pair of mesenteries (Bayer et al. 1983).
It is inferred that the mesenteries increase surface area for respiration and especially absorption of food. They also provide support. Perhaps for these reasons, anthozoans of some species can grow much larger than polyps in any other class of Cnidaria -- to a diameter of 1.25 m for the "giant" sea anemone Stichodactyla mertensii (see Dunn 1981) (Title Figure 1). The gametogenic tissue of anthozoans lies between the longitudinal retractor muscle and the edge of a mesentery (but not all mesenteries of an individual may be gametogenic); gametes are derived from endodermal cells (eg Wedi and Dunn 1983). Polyps of some anthozoan species are gonochoric, those of some species are hermaphroditic, and those of still others change sex during their lifetime. Anthozoans of many species broadcast their gametes, but brooding of embryos within the coelenteron and attached to the external surface of the polyp is known (Fautin et al. 1989).
Variation within Anthozoa
Molecular data (France et al. 1996; Berntson et al. 1999; see Discussion of Phylogenetic Relationships below) support the division of the class into two groups, which are externally distinguished by the number and form of a polyp's tentacles. Subclass Alcyonaria (= Octocorallia) includes animals such as soft corals, sea fans, sea pens, and blue corals. Each polyp has eight tentacles that are pinnately branched (Title Figure 2); there are also eight complete mesenteries, so the space between each two mesenteries extends into one tentacle. The actinopharynx of each polyp has a single siphonoglyph. Alcyonarians of most species form colonies and skeletons. Mariscal (in Fautin and Mariscal 1991) proposed Alcyonaria be elevated to the rank of class. Extant members of subclass Zoantharia (= Hexacorallia) include sea anemones, "stony" corals, and black corals. Despite the name of the subclass, almost no members have six tentacles. Rather, zoantharians typically have (approximately) a multiple of six mesenteries and tentacles. Depending on the taxon, one or more than one tentacle may arise from each intermesenterial space.
Zoantharians fall into two clear groups: those in which mesenteries occur singly (Antipatharia [black corals] and Ceriantharia [tube anemones]), and those in which the mesenteries develop in pairs (all the others; see Figure 1). Black corals are the only exclusively colonial zoantharians.
Discussion of Phylogenetic Relationships
Class Anthozoa is traditionally considered to have two or three subclasses. Hyman (1940) divided the class into Alcyonaria and Zoantharia, based largely on polyp symmetry and tentacle form and number. Wells and Hill (1956) recognized a third subclass, Ceriantipatharia (listed also by Dunn 1982), based on similarity of the ceriantharian larval stage to the antipatharian polyp, on the very weak mesentery musculature in both groups, and on the insertion of new mesenterial couples only in the dorsal intermesenterial space of members of both orders. Evidence from morphology (Hand 1966), nematocysts (Hand 1966, Schmidt 1974), and DNA (France et al. 1996, Song and Won 1997, Berntson et al. 1999) does not support the monophyly of the Ceriantharia and Antipatharia. Indeed, Hand (1966) suggested division of Anthozoa into four subclasses (Antipatharia, Ceriantharia, Zoantharia, and Alcyonaria) could be justified. While the most complete molecular data available (Berntson et al. 1999) do not support the monophyly of the Ceriantharia and Antipatharia, placement of the Ceriantharia remains uncertain. The Ceriantharia may merit subclass status but more data are necessary to determine its phylogenetic position (Berntson et al. 1999).
We have chosen to follow Hyman (1940) in treating Antipatharia and Ceriantharia as orders of subclass Zoantharia. The zoantharian lineage has been interpreted as derived from the Alcyonaria, based on development (e.g. Hand 1966) and nematocysts (Schmidt 1974), but DNA sequences obtained thus far (Song et al. 1994, Bridge et al. 1995, Chen et al. 1995, France et al. 1996, Song and Won 1997, Berntson et al. 1999) support a sister relationship between Alcyonaria and Zoantharia.
Anthozoan Relationships Forum
The phylogenetic relationships of anthozoan subgroups are currently the subject of much debate. In the above Discussion of Phylogenetic Relationships, Daphne Fautin and Sandra Romano have given a summary of the most important issues. Many open questions remain, and there is some controversy about the conclusions that can be drawn from the data collected to date. In order to adequately represent alternative points of view in this discussion, we have established an Anthozoan Relationship Forum on a linked page.
References
Bayer, F. M. 1956. Octocorallia. Pp. F166-F230 in: R. C. Moore (ed.), Treatise on Invertebrate Paleontology Part F: Coelenterata. Geological Society of America and University of Kansas Press, Lawrence.
Bayer, F. M., M. Grasshoff, and J. Verseveldt. 1983. Illustrated trilingual glossary of morphological and anatomical terms applied to Octocorallia. E. J. Brill / Dr. W.Backhuys, Leiden. 75 pp.
Berntson, E. A., S. C. France, and L. S. Mullineaux. 1999. Phylogenetic relationships within the Class Anthozoa (Phylum Cnidaria) based on nuclear 18S rDNA sequences. Molecular Phylogenetics and Evolution 13: 417-433.
Bridge, D., C. W. Cunningham, R. deSalle, and L. W. Buss. 1995. Class-level relationships in the phylum Cnidaria: Molecular and morphological evidence. Molecular Biology and Evolution 12: 679-689.
Chen, C. A., D. M. Odorico, M. ten Lohuis, J. E. N. Veron, and D. J. Miller. 1995. Systematic relationships within the Anthozoa (Cnidaria: Anthozoa) using the 5'-end of the 28S rDNA. Molecular Phylogeny and Evolution 4: 175-183.
Dunn, D. F. 1981. The clownfish sea anemones: Stichodactylidae (Coelenterata: Actiniaria) and other sea anemones symbiotic with pomacentrid fishes. Transactions of the American Philosophical Society 71(1): 1-115.
Dunn, D. F. 1982. Cnidaria. Pp. 669-706 in S. P. Parker (ed.), Synopsis and Classification of Living Organisms, volume 1. McGraw-Hill Book Company, New York and other cities.
Fautin, D. G. and R. N. Mariscal. 1991. Cnidaria: Anthozoa. Pp. 267-358 in F. W. Harrison and J. A. Westfall (eds.), Microscopic Anatomy of Invertebrates, volume 2: Placozoa, Porifera, Cnidaria, and Ctenophora. Wiley-Liss, Inc., New York and other cities.
Fautin, D. G., J. G. Spaulding, and F.-S. Chia. 1989. Cnidaria. Pp. 43-62 in K. G. Adiyodi and R. G. Adiyodi (eds.), Reproductive Biology of Invertebrates, volume 4, part A (Fertilization, Development, and Parental Care). Oxford and IBH Publ. Co., New Delhi.
France, S. C., P. E. Rosel, J. E. Agenbroad, L. S. Mullineaux, and T. D. Kocher. 1996. DNA sequence variation of mitochondrial large-subunit rRNA provides support for a two subclass organization of the Anthozoa (Cnidaria). Molecular Marine Biology and Biotechnology 5: 15-28.
Hand, C. 1966. On the evolution of the Actiniaria. Pages 135-146 in W. J. Rees (ed.), The Cnidaria and their Evolution. Academic Press, London and New York.
Hyman, L. 1940. The Invertebrates. McGraw-Hill, New York. 726 pp.
Hyman, L. H. 1956. Morphology of living coelenterates. Pp. F10-F20 in: R. C. Moore (ed.), Treatise on Invertebrate Paleontology Part F: Coelenterata. Geological Society of America and University of Kansas Press, Lawrence.
Oliver, W. A. J. and A. G. Coates. 1987. Phylum Cnidaria. Pp. 140-193 in R. S. Boardman, A. H. Cheetham, and A. J. Rowell (eds.), Fossil Invertebrates. Blackwell Scientific Publications, Palo Alto, California.
Schmidt, H. 1974. On evolution in the Anthozoa. Proceedings of the Second International Coral Reef Symposium 1: 533-560.
Schuchert, P. 1993. Phylogenetic analysis of the Cnidaria. Zeitschrift fuer zoologische Systematik und Evolutionsforschung 31: 161-173.
Shick, J. M. 1991. A Functional Biology of Sea Anemones. Chapman and Hall, London and other cities. 395 pp.
Song, J.-I., W. Kim, E. K. Kim, and J. Kim. 1994. Molecular phylogeny of anthozoans (phylum Cnidaria) based on the nucleotide sequences of 18S rRNA gene. Korean Journal of Zoology 37: 343-351.
Song, J.-I. and J. H. Won. 1997. Systematic relationship of the anthozoan orders based on the partial nuclear 18S rRNA sequences. Korean Journal of Biological Science 1: 43-52.
Wedi, S. E. and D. F. Dunn. 1983. Gametogenesis and reproductive periodicity of the subtidal sea anemone Urticina lofotensis (Coelenterata: Actiniaria) in California. Biological Bulletin 165: 458-472.
Wells, J. W. 1956. Scleractinia. Pp. F328-F440 in: R. C. Moore (ed.), Treatise on Invertebrate Paleontology Part F: Coelenterata. Geological Society of America and University of Kansas Press, Lawrence.
Wells, J. W. and D. Hill. 1956. Anthozoa - general features. Pp. F161-F165 in: R. C. Moore (ed.), Treatise on Invertebrate Paleontology Part F: Coelenterata. Geological Society of America and University of Kansas Press, Lawrence.
Title Illustrations

| Scientific Name | Stichodactyla mertensii |
|---|---|
| Comments | The "giant" sea anemone, a member of subclass Zoantharia. |
| Specimen Condition | Live Specimen |
| Copyright | © 1999 Gerald R. Allen |
About This Page
Creation of this page was supported by NSF grant DEB95-21819 (PEET: Partnerships for Enhancing Expertise in Taxonomy) to DGF. Technical assistance was rendered by Chad Campbell and Adorian Ardelean. S. Romano acknowledges support from an NIH grant to R. H. Richmond in writing this page.
Daphne G. Fautin

University of Kansas, Lawrence, Kansas, USA
Sandra L. Romano

University of the Virgin Islands, St. Thomas, USVI
Page copyright © 2000 and
All Rights Reserved.
- First online 04 October 2000
- Content changed 03 October 2000
Citing this page:
Fautin, Daphne G. and Sandra L. Romano. 2000. Anthozoa. Sea Anemones, Corals, Sea Pens. Version 03 October 2000. http://tolweb.org/Anthozoa/17634/2000.10.03 in The Tree of Life Web Project, http://tolweb.org/










 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site