Aspidytidae
Aspidytes
Cliff Water Beetles
Ignacio Ribera, Michael Balke, and Rolf Beutel- Aspidytes niobe
- Aspidytes wrasei
Introduction
In 1995 the German Coleopterist David Wrase collected some specimens of an unknown dytiscoid at the base of a rock surface in Shaanxi, China. The specimens were eventually sent to the Naturhistorisches Museum in Wien (NMW) and preliminarily diagnosed as a possible new genus of Noteridae. On a collecting trip to the Western Cape region in 2001 Ignacio Ribera and Alexandra Cieslak collected three specimens of what looked like a new noterid genus in the field, but one that was unable to swim: it had no trace of swimming hairs, and paddled inefficiently in the pooter half full with water from sucking directly over the hygropetric surface in which they were found. A more careful study back at The Natural History Museum in London (BMNH), including the first molecular data, revealed that these specimens were not directly related to Noteridae or Dytiscidae, and represented a new family that was described under the name Aspidytidae, or “diving shield” (Ribera et al. 2002). After sending a paratype to the NMW with the hint of comparing it with the new “noterid” from China that had been examined during a previous visit, the link was made and the second species of the genus Aspidytes was described in Balke et al. (2003). Despite the distinct morphological differences between the two species it was preferred to keep them under the same genus, waiting for the prospect of the discovery of additional species of Aspidytidae.
After the description of the genus, David T. Bilton collected additional adult specimens of A. niobe plus the larvae at the type locality (Alarie & Bilton, 2005). So far the species is known only from some rocky outcrops in a restricted area in the Western Cape region of South Africa. As far as we know, the Chinese species A. wrasei has not been collected again.
Characteristics
Adults
Total length 4.8–7.0 mm. Body streamlined, convex dorsally, without pronoto-elytral angle. Body and appendages ferrugineous to black; cuticle shiny.

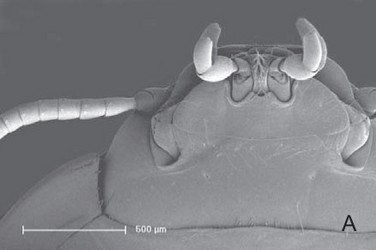
Aspidytes niobe, ventral view of head. © 2011 Michael Balke
Head prognathous, distinctly shortened, wedge-shaped in lateral view. Posterior part of head distinctly retracted into prothorax, but without narrowed neck region. Clypeus distinctly narrowing towards labral articulation; frontoclypeal suture broadly interrupted medially. Clypeolabral suture almost straight. Labrum transverse, with wide median concavity. Antennae without pubescence or longer setae; inserted laterally, anterad to compound eyes; articulation not visible from above; scapus with globular basal piece and globular distal part separated by a distinct circular incision; distal part largely enclosing small pedicellus. Maxilla inserted in maxillary groove between submentum and mentum and the compound eye; galea palp-like, 2-segmented; palp 5-segmented, fairly short, scarcely overtopping labial palp.

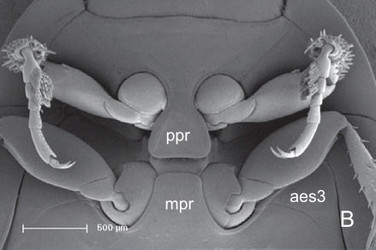
Aspidytes niobe, ventral view of prothorax and mesothorax. © Michael Balke
Pronotum laterally evenly rounded, with distinct lateral and posterior beads; inflected margin of pronotum distinctly widening posteriorly; posterior edge with dense row of hairs. Prosternal process well developed, posteriorly rounded (A. wrasei) or truncate, with rounded posterolateral edges (A. niobe). Procoxal cavities open, with sclerotised, rufous internal bridge. Foreleg fairly short; procoxae globular, with short ventral condyle; femora with short row of stiff hairs at anterior margin (profemoral cleaning device); tibia with truncate apex and two strong terminal spurs; external spur slightly stronger; tarsomeres 1–4 short; tarsomere 5 ca. 2.5 times as long as basal tarsal segments; male tarsomeres 1 and 2 laterally slightly dilated and ventrally with numerous adhesive hairs with minute apical discs; claws equal. Mesothorax slightly shorter than prothorax. Scutellar shield exposed. Elytra with lateral bead extending to apex; epipleura broad anteriorly, strongly narrowing towards abdominal apex; with polygonal meshes of different size and few punctures. Mesocoxal cavity laterally bordered by mesal edge of epimeron and narrow apical part of metathoracic anepisternum. Middle legs similar to forelegs; femur without tuft of spines; tibia and tarsus slightly longer; tibia only very slightly extended distally; tarsomeres 1 and 2 of males laterally slightly dilated and ventrally with numerous adhesive hairs with minute apical discs. Metathorax slightly longer than prothorax. Metasternal process well developed, with rounded anterolateral edges. Transverse suture present as faintly impressed line and internal ridge or as internal ridge only (A. niobe); about as long as width of internal lamina of metacoxa, separating posterior katepisternum from anterior praeepisternal part of ventrite. Median longitudinal suture (= discriminal line or discrimen) very short, not surpassing posterior half of katepisternum. Metacoxa moderately extended, slightly longer than ventrite; lateral margin broadly contiguous with epipleural margin; mesal walls extensively fused, forming large intercoxal septum; anterior paramedian angle absent; inner lamina and coxal plates distinct, broadened posteriorly, distinctly curved outwards anteriorly; metatrochanter larger than pro- and mesotrochanter; tibia and tarsus longer than those of middle leg, appearing thin and longish. Wings well developed (examined in A. niobe), with distinct oblongum cell and moderately elongate medial setal binding patch (Beutel et al. 2006).


Aspidytes niobe, ventral view of thorax. © 2011 Michael Balke
Abdomen with six exposed ventrites; length decreasing from III to VI; sternite II only visible laterad of metacoxa; sternites III and IV partly fused, but with very distinct separating suture. Lateral bead present on all sternites, most prominent along lateral margin of sternite VII. Shape of sternite VII roughly semicircular, evenly rounded posteriorly, and devoid of modifications of its surface or hind margin. Male genitalia of A. wrasei with median lobe of aedeagus evenly curved; symmetrical parameres of roughly triangular shape; apex stylus-like, with few short setae anteriorly; margin devoid of setae (see Balke et al. 2003). A. niobe with median lobe of aedeagus composed of several lobes and membranous sacs; parameres broadly oval; apex rounded with few short setae. Gonocoxae (= genital appendages IX) of females of A. wrasei roughly rectangular in ventral view, with very slightly curved lateral margins and blunt apex. Gonocoxa of A. niobe elongate in ventral view, with apex broadly rounded. Spermatheca without any obvious modifications in both species; spermathecal gland always absent.
Larvae
Only the larva of A. niobe is known (Alarie & Bilton 2005; Balke et al. 2005). Total length of mature larvae (excl. urogomphi) ca. 7 mm. Robust, almost onisciform anteriorly, but abdomen more subcylindrical towards apical segment IX. Head and body strongly pigmented on dorsal side. Cuticle smooth. Sclerites set with short setae and also setae of medium length.
Head prognathous, very slightly inclined; posteriorly retracted into prothorax but without distinct neck region. Moderately flattened, slightly broader than long, distinctly narrower than prothorax. Greatest width (ca. 1 mm) at posterior 1/3 of head; head capsule abruptly narrowing towards foramen occipitale and gradually narrowing towards clypeal region. Six moderately sized stemmata present posterad of antennal articulatory region, nearly arranged in a circle. Frontal suture (=frontal arms) present, lyriform, but with very indistinct indentation. Frons distinctly elongated posteriorly. Coronal suture accordingly shortened, ca. 1/3 as long as entire head capsule. Labrum fused with frontoclypeus. Adnasalia (=epistomal lobes) distinct, slightly asymmetric; with several long setae and dense brush of microtrichia along mesal part of anterior margin and ventral surface. Nasale without teeth, separated from adnasalia by very deep grooves. Antennae 4-segmented. Mandibles moderately long, with fairly broad basal part and mesal groove bordered by two cutting edges, one of them toothed; retinaculum present; mola and prostheca missing. Maxilla inserted at anteroventral margin of head; cardo steep, hardly visible in ventral view; galea palp-like, 2-segmented; lacinia absent; palp 3–segmented. Submentum completely fused with gula posteriorly and with ventrolateral wall of head capsule laterally; mentum membranous, short; prementum short, without ligula; palpiger well developed; palp ventrally directed, 2-segmented; both palpomeres about equally long, but basal one distinctly wider. Gula moderately long and broad. Tentorium with long caudal arms.
Prothorax largest segment of body; cuticle smooth and shiny, with numerous short setae inserted in small pores and longer setae along lateral and anterior margin of posterior collar. Pronotum shield-like, rounded anteriorly and laterally, with a distinct bead along lateral margin; epipleura, i. e., inflected part of pronotum (paratergal lobe) distinctly developed; posterior collar fairly broad, with parallel longitudinal riffles. Procoxae large and prominent, cone-shaped; distinct lateral concavity for reception of profemora in repose delimited by sharp anterior edge and less conspicuous posterior edge; anterior edge with several long setae; trochanter well developed; femur rather short, ca. 2 times as long as wide, with 4 spines along ventral edge; tibia shorter and narrower, moderately extended distally, with several apical setae; tarsus shorter than tibia, cylindrical, with two equal, strongly developed claws; single seta present between claws. Mesothorax shorter than prothorax, otherwise similar. Trochanters and femora slightly larger than on prothorax. Metathorax very similar to mesothorax. Coxae larger; femora and tibiae slightly longer.
Abdominal segment I approximately half as long as metathorax; with lateral bead, narrow paratergal lobes and posterior collar; spiracle small, located on lateral part of tergite. Sternal part with two distinct oblique ridges. Tergites of segments II–VIII laterally fused with sternites, thus forming ring-shaped structure. Segments II–V distinctly longer than I, about equally broad. Segments VI and VII shorter; VI narrower than V, and VII narrower than VI; VII about as long as V and distinctly narrowing posteriorly. Strongly developed urogomphi inserted on segment VIII ventrolaterally, enclosing small but distinctly developed segment IX (visible in ventral and dorsal view); basal segment of urogomphi ca. 1.5 times as long as segment VIII; with broad bases almost contiguous ventromedially; evenly tapering towards apex; with several long setae; distal segment very slender, cylindrical, slightly more than half as long as basal segment; with long setae inserted on apex.
Pupa and eggs unknown.
Discussion of Phylogenetic Relationships
Aspidytes clearly belongs to Dytiscoidea, as shown by both molecular and morphological data (Ribera et al. 2002; Balke et al. 2005; Beutel et al. 2006). Some synapomorphies of Dytiscoidea are the extensive fusion of the mesal metacoxal walls, the loss of two of three metathoracic furcacoxal muscles, and the loss of the abdominal segment X in larvae. A feature apparently plesiomorphic compared to other dytiscoid groups is the incomplete reduction of the larval abdominal segment IX (Alarie & Bilton 2005).
Within Dytiscoidea, the relationships among families Dytiscidae, Hygrobiidae, Amphizoidae and Aspidytidae have still not been fully clarified. The most complete analyses to date (Balke et al. 2005), with fifty-three morphological characters of adults and larvae and ca. 6 Kb from six nuclear and mitochondrial genes, placed Aspidytes as sister to Amphizoa, although with relatively low support.
There is yet no data to allow an estimation of the age of the separation between the two known species of Aspidytes, with widely disjunct distributions.
Ecology, life cycle
The specimens of Apidytes wrasei were collected among stones and various plants at the base of a vertical hygropetric surface (Balke et al. 2003).


Figure 1. Aspidytes niobe collection site in South Africa (ca. 20 km NE Wellington).
Aspidytes niobe is known from several rocky outcrops covered with a permanent water film a few millimeters deep (Figures 1, 2). Adults and larvae are usually found resting on vertical or almost vertical surfaces devoid of vegetation, but when disturbed are able to scuttle rapidly over the surface in search of cover under the algae or other vegetation (Alarie & Bilton 2005).


Figure 2. Aspidytes niobe collection site in South Africa (Michell's Pass, Ceres).
The two type localities were the only wet spots in the whole area at the end of the summer of 2001 (24th March), an exceptionally dry year. They were together with a diverse and abundant fauna of aquatic Coleoptera, many of them showing apparent morphological adaptations to a madicolous habit: Delevea bertrandi Reichardt (Torridincolidae), Canthyporus aenigmaticus Biström & Nilsson, C. nebulosus Omer-Cooper, Africophilus sp. (Dytiscidae), Coelometopon blinkwater Perkins, Oomtelecon sebastiani Perkins, Pneuminion velamen Perkins (Hydraenidae) plus some unidentified Dryopidae, Elmidae and Hydrophilidae.
Both adults and larvae are probably predaceous, although there is no direct evidence of their feeding habits. Adults of A. wrasei were found in late summer (August), and adults of A. niobe have been found from Spring to late Summer (September to March). Larvae were absent in late summer (March), but common in September (Alarie & Bilton 2005; D. T. Bilton pers. communication 2010).
Molecular data
Molecular data from the holotype and one paratype of A. niobe are available and were used in different studies to establish the phylogenetic placement of the family (Ribera et al. 2002; Balke et al. 2005; Beutel et al. 2006).
| Voucher | Cox1 | rrnL | 18S | Cob | rrnS | H3 |
|---|---|---|---|---|---|---|
| IR600 / BMNH 681813 (Holotype) | AY071808 | AY071782 | AY071794 | AY745657 | AY745640 | AY745674 |
| IR627 (Paratype) | AY071809 | AY071783 | EF670233 |
The complete mitochondrial genome of a larva of A. niobe collected by D.T. Bilton in one of the type localities (Mitchell’s pass, Ceres) was sequenced (Pons et al. 2010). It is available in GenBank with accession number NC_012139
References
Alarie, Y., Bilton, D.T. 2005. Larval morphology of Aspidytidae (Coleoptera : Adephaga) and its phylogenetic implications. Annals of the Entomological Society of America 98: 417-430.
Balke, M., Ribera, I., Beutel, R.G. 2003. Aspidytidae: on the discovery of a new beetle family: detailed morphological analysis, description of a second species, and key to fossil and extant adephagan families (Coleoptera). In: Water beetles of China. Volume 3. (eds. Jaech MA, Ji L), pp. 53-66. Zoologisch - Botanische Gesellschaft.
Balke, M., Ribera, I., Beutel, R.G. 2005. The systematic position of Aspidytidae, the diversification of Dytiscoidea (Coleoptera, Adephaga) and the phylogenetic signal of third codon positions. Journal of Zoological Systematics and Evolutionary Research 43: 223-242.
Beutel, R.G., Balke, M. & Ribera, I. 2010. 3.1. Aspidytidae Ribera, Beutel, Balke and Vogler, 2002. In: Leschen, R.A.B., Beutel, R.G. & Lawrence, J.F. (eds.): Handbook of Zoology, Arthropoda: Insecta. Coleoptera, Beetles. Vol. 2: Morphology and Systematics (Elateroidea, Bostrichiformia, Cucujiformia partim). Walter de Gruyter, Berlin. pp. 21-28.
Beutel, R.G., Balke, M., Steiner, W.E. 2006. The systematic position of Meruidae (Coleoptera, Adephaga) and the phylogeny of the smaller aquatic adephagan beetle families. Cladistics 22: 102-131.
Beutel, R.G., Ribera, I., Bininda-Emonds, O.R.P. 2007. A genus-level supertree of Adephaga (Coleoptera). Organisms Diversity & Evolution 7: 255-269.
Nilsson, A.N., van Vondel, B.J. 2005. Volume 7: Amphizoidae, Aspidytidae, Haliplidae, Noteridae and Paelobiidae (Coleoptera, Adephaga). In: World catalogue of insects, pp. 1-171. Apollo Books.
Pons, J., Ribera, I., Bertranpetit, J. & Balke, M. 2010. Nucleotide substitution rates for the full set of mitochondrial protein coding genes in Coleoptera. Molecular Phylogenetics and Evolution, 56: 796-807.
Ribera, I., Beutel, R.G., Balke, M., Vogler, A.P. 2002. Discovery of Aspidytidae, a new family of aquatic Coleoptera. Proceedings of the Royal Society of London Series B-Biological Sciences 269: 2351-2356.
Ribera, I., Bilton, D.T. 2009. Chapter 7: Aspidytidae. In: Stals, R. & de Moor, I.J. (eds). Guides to the Freshwater Invertebrates of Southern Africa. Volume 10: Coleoptera. Water Research Commission Report No. TT 320/07, Pretoria, South Africa, pp. 85-88.
Title Illustrations

| Scientific Name | Aspidytes niobe |
|---|---|
| Specimen Condition | Dead Specimen |
| Identified By | Ignacio Ribera |
| Life Cycle Stage | adult |
| View | dorsal |
| Copyright |
© 2011 Ignacio Ribera

|
About This Page
Ignacio Ribera

Museo Nacional de Ciencias Naturales, Madrid, Spain
Rolf Beutel

Inst. f. spez. Zool. u. Evolut.-Biol.
Correspondence regarding this page should be directed to Ignacio Ribera at , Michael Balke at , and Rolf Beutel at
Page copyright © 2011 Ignacio Ribera, , and Rolf Beutel
 Page: Tree of Life
Aspidytidae. Aspidytes. Cliff Water Beetles.
Authored by
Ignacio Ribera, Michael Balke, and Rolf Beutel.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Aspidytidae. Aspidytes. Cliff Water Beetles.
Authored by
Ignacio Ribera, Michael Balke, and Rolf Beutel.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
- First online 03 March 2011
- Content changed 03 March 2011
Citing this page:
Ribera, Ignacio, Michael Balke, and Rolf Beutel. 2011. Aspidytidae. Aspidytes. Cliff Water Beetles. Version 03 March 2011. http://tolweb.org/Aspidytes/29299/2011.03.03 in The Tree of Life Web Project, http://tolweb.org/











 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site