Braconidae
James B. Whitfield, Won-Young Choi, Alejandro A. Valerio, Josephine Rodriguez, and Andrew R. Deans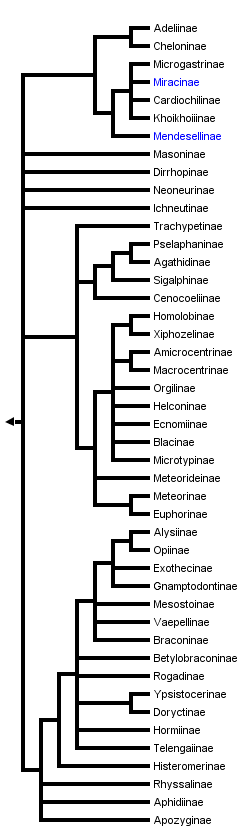


This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
The Braconidae constitute one of the most species-rich families of insects. Although tropical faunas are still relatively poorly understood at the species level, most taxonomists in this group would agree that a rough, probably highly conservative, estimate of 40-50,000 species worldwide is reasonable as an extrapolation from the current described number of roughly 12,000 species. Among extant groups, the sister group of the Braconidae is the Ichneumonidae, an equally enormous group (Sharkey and Wahl, 1992; Quicke et al. 1999).
The family appears to date from early Cretaceous (assuming Eobracon is properly assigned to family - Rasnitsyn, 1983; Whitfield, 2002), diversifying extensively in the mid to late Cretaceous and early Tertiary, when flowering plants and their associated holometabolous herbivores, the main hosts for braconid parasitoids, radiated (Basibuyuk et al., 1999; Quicke et al., 1999; Belshaw et al., 2000; Whitfield, 2002). The species richness of the family is matched by a morphological diversity virtually unrivalled among the Hymenoptera. They range in size from approximately 1 mm in length to 3-4 cm (not counting the ovipositor, which in some species can be several times as long as the body).


Figure 1. Microgastrine braconid adult female (length approx. 2 mm). Image copyright © 2004 James B. Whitfield
Some of the groups are parts of extensive Müllerian mimicry complexes (Quicke, 1986), and exhibit striking color patterns (some of which are recurrent within regions), while others are among the most inconspicuous of Hymenoptera. The braconids display a bewildering array of wing venation patterns and body forms to stymie the beginning student. Female external genitalia (ovipositor mechanisms) vary considerably intraspecifically and are widely used for species discrimination;


Figure 2. Ovipositior mechanism of Andesipolis, for reaching into plant tissue. Image copyright © 2004 Won-Young Choi

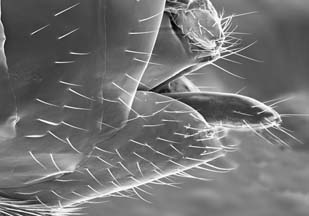
Figure 3. Ovipositor mechanism of Cotesia, for inserting eggs into exposed caterpillars. Image copyright © 2004 Won-Young Choi
while the male genital capsules tend to be somewhat more conservative and have been underutilized relative to other insect groups.


Figure 4. Male genital capsule of Andesipolis, from a relatively basal lineage of Braconidae. Image copyright © 2004 Won-Young Choi


Figure 5. Male genital capsule of Hypomicrogaster, from a relatively derived (microgastroid) lineage.
Image copyright © 2004 Josephine Rodriguez
Characteristics
The vast majority of braconids are primary parasitoids of other insects, especially upon the larval stages of Coleoptera, Diptera, and Lepidoptera but also including some hemimetabolus insects (aphids, Heteroptera, Embiidina). As parasitoids they almost invariably kill their hosts, although a few only cause their hosts to become sterile and less active. Both external and internal parasitoids are common in the family, and the latter forms often display elaborate physiological adaptations for enhancement of larval survival within host insects, including the co-option of endosymbiotic viruses for compromising host immune defenses (Stoltz and Vinson, 1979; Stoltz, 1986; Whitfield, 1990; Beckage, 1993, Stoltz and Whitfield, 1992; Whitfield, 2002; Whitfield and Asgari, 2003).
Early larval development in braconids has also yielded surprises, such as the discovery of relatively closely related genera that differ in such import aspects as syncitial versus holoblastic cleavage, normally characterizing major animal phyla (Grbic and Strand, 1998; Grbic, 2000)! Parasitism of adult insects (especially of Hemiptera and Coleoptera) is also known, and members of two subfamilies (Mesostoinae and Doryctinae) form galls on plants (Infante et al., 1995; Austin and Dangerfield, 1998). Several excellent general reviews of braconid biology are available (Matthews, 1974; Shaw and Huddleston, 1991; Shaw, 1995; Wharton, 1993a).


Figure 6. Microplitis ceratomiae larvae emerging from sphingid caterpillar.
Image copyright © 2004 James B. Whitfield


Figure 7. Sphingid caterpillar with cocoons of Cotesia congregata.
Image copyright © 2004 Sydney A. Cameron
Discussion of Phylogenetic Relationships
Early attempts to explore the phylogeny of the major groups of Braconidae (e. g., Tobias, 1967; van Achterberg, 1984) were largely intuitive. Quicke and van Achterberg (1990) made the first large-scale effort to estimate braconid phylogeny using a large data set of morphological characters, and computer-assisted parsimony analysis. Although many aspects of this study have been in dispute (Wharton et al., 1992; van Achterberg and Quicke, 1992), it has stimulated a number of further attempts at resolving the phylogeny of subset groups of the Braconidae, using morphology as well as molecular data (e. g. Belshaw and Quicke, 1997; Whitfield, 1997; Belshaw et al., 1998; Dangerfield et al., 1999; Dowton et al., 1998; Dowton, 1999; Mardulyn and Whitfield, 1999; Quicke and Belshaw, 1999; Quicke et al., 1999; Belshaw et al., 2000; Kambhampati et al., 2000; Sanchis et al., 2000; Belshaw et al., 2001; Dowton et al., 2002; Whitfield et al., 2002; Chen et al. 2003).
The phylogeny used as the backbone here for accessing the subfamilies is adapted and pasted together, with a great deal of conservative poetic license, from these recent studies. It should not be taken all that seriously, as help is on the way in the form of continuing morphological and molecular phylogenetic studies. The reader is encouraged to consult the literature cited above for more in-depth analysis.
Classification
The higher classification of the Braconidae has been a matter of much dispute. Approximately 40 subfamilies are generally recognized, several of them newly discovered within the last 15 years (Mason, 1983; Quicke, 1987; Whitfield and Mason, 1994). Several subfamilies (e.g., Aphidiinae, Alysiinae, Apozyginae) have at one time or another been recognized as separate families. In general there is agreement on the basic subfamily or tribal groupings, with the exception of the "hormiine" and "exothecine" groups of genera (Whitfield, 1992; Quicke, 1993; Wharton, 1993b), but disagreement on the ranking or inclusiveness of some groups, since our understanding of the phylogeny of the family is not yet robust. There is a general perception that the number of recognized subfamilies has become inflated, while the tribal rank has been underutilized. An attempt has been made to address this problem (Wharton, 2001), but requires further phylogenetic testing.
Identification Guides
Keys to the world subfamilies and general introductions to the literature are available in van Achterberg (1993) and Sharkey (1993). A new manual to the genera of the New World has been produced (Wharton et al., 1997 - Spanish version 1998), including individual subfamily chapters contributed by a number of the world's braconid specialists. Interactive versions of the keys in this manual are available online. van Achterberg (1997) has published the CD-ROM Braconidae. An illustrated Key to all Subfamilies.
References
van Achterberg, C. 1984. Essay on the phylogeny of Braconidae (Hymenoptera: Ichneumonoidea). Entomol. Tidskr. 105: 41-58.
van Achterberg, C. 1993. Illustrated key to the subfamilies of the Braconidae (Hymenoptera: Ichneumonoidea. Zool. Verhand. 283: 1-189.
van Achterberg, C. 1997. Braconidae: an illustrated key to all subfamilies. CD-ROM produced by ETI and available from Springer-Verlag, NY.
van Achterberg, C. and D. L. J. Quicke. 1992. Phylogeny of the subfamilies of the Braconidae: a reassessment assessed. Cladistics 237-264.
Austin, A. D. and P. C. Dangerfield (1998) Biology of the Mesostoa kerri Austin and Wharton (Insecta: Hymenoptera: Braconidae: Mesostoinae), an endemic Australian wasp that causes stem galls on Banksia marginata Cav. Austral. J. Bot. 46:559-569.
Basibuyuk, H. H., A. P. Rasnitsyn, K. van Achterberg, M. G. Fitton and D. L. J Quicke. 1999. A new, putatively primitive Cretaceous fossil braconid subfamily from New Jersey amber (Hymenoptera: Braconidae). Zool. Script. 28: 211-214.
Beckage, N. E. 1993. Games parasites play: the dynamic roles of proteins and peptides in the relationship between parasite and host. Chapter 2, pp. 25-57 in: Beckage, N. E., S, N. Thompson and B. A. Federici, eds., Parasites and Pathogens of Insects, Volume 1: Parasites, Academic Press, San Diego.
Belshaw,R., M. Dowton, D. L. J. Quicke and A. D. Austin. 2000. Estimating ancestral geographical distributions: a Gondwanan origin for aphid parasitoids? Proc. R. Soc. Lond., B, Biol. Sci. 267: 491-496.
Belshaw, R., M. Fitton, E. Herniou, C. Gimeno and D. L. J. Quicke. 1998. A phylogenetic reconstruction of the Ichneumonoidea (Hymenoptera) based on the D2 variable region of 28S ribosomal RNA. Syst. Entomol. 23: 109-123.
Belshaw, R., C. Lopez-Vaamonde, N. Degerli and D. L. J. Quicke. 2001.Paraphyletic taxa and taxonomic chaining: evaluating the classification of braconine wasps (Hymenoptera: Braconidae) using 28S D2-3 rDNA sequences and morphological characters. Biol. J. Linn. Soc. 73: 411-424.
Belshaw, R. and D. L. J. Quicke. 1997. A molecular phylogeny of the Aphidiinae (Hymenoptera: J. Braconidae). Mol. Phylo. Evol. 7: 281-293.
Chen,X.-X., M. -H. Piao, Whitfield and J. -H. He. 2003. A molecular phylogeny of the subfamily Rogadinae (Hymenoptera: Braconidae) based on the D2 variable region of 28S ribosomal RNA. Acta Entomologica Sinica 46: 209-217.
Dangerfield, P. C., A. D. Austin and J. B. Whitfield. 1999. Systematics of the world genera of Cardiochilinae (Hymenoptera: Braconidae). Invert. Tax. 13: 917-976.
Dowton, M. 1999. Relationships among the cyclostome braconid (Hymenoptera: Braconidae) subfamilies inferred from a mitochondrial tRNA gene rearrangement. Mol. Phylo. Evol. 11: 283-287.
Dowton, M., A. D. Austin and M. F. Antolin. 1998. Evolutionary relationships among the Braconidae (Hymenoptera: Ichneumonoidea) inferred from partial 16S rDNA gene sequences. Insect Mol. Biol. 7: 129-150.
Dowton, M., R. Belshaw, A. D. Austin and D. L. J. Quicke. 2002. Simultaneous molecular and morphological analysis of braconid relationships (Insecta: Hymenoptera: Braconidae) indicates independent mt-tRNA gene inversions within a single wasp family. J. Mol. Evol. 54: 210-226.
Grbic, M. 2000. "Alien" wasps and evolution of development. BioEssays 22: 920-932.
Grbic, M. and Strand, M. R. 1998. Shifts in the life history of parasitic wasps correlate with pronounced alterations in early development. Proceedings of the National Academy of Sciences of the USA 95: 1097-1101.
Infante, F., P. Hanson and R. Wharton. 1995. Phytophagy in the genus Monitoriella (Hymenoptera: Braconidae) with description of new species. Ann. Entomol. Soc. Amer. 88: 406-415.
Kambhampati,S., Voelkl,W. and Mackauer,M. 2000. Phylogenetic relationships among genera of Aphidiinae (Hymenoptera:Braconidae) based on DNA sequence of the mitochondrial 16S rRNA gene. Syst. Entomol. 25: 437-445.
Mardulyn, P. and J. B. Whitfield. 1999. Phylogenetic signal in the COI, 16S and 28S genes for inferring relationships among genera of Microgastrinae (Hymenoptera: Braconidae): evidence of a high diversification rate in this group of parasitoids. Mol. Phylo. Evol. 12: 282-294.
Mason, W. R. M. 1983. A new South African subfamily related to Cardiochilinae (Hymenoptera: Braconidae). Contrib. Amer. Entomol. Inst. 20: 49-62.
Matthews, R. W. 1974. Biology of Braconidae. Ann. Rev. Entomol. 19: 15-32.
Quicke, D. L. J. 1986. Preliminary notes on homeochromatic associations within and between the afrotropical Braconinae (Hym., Braconidae) and Lamiinae (Col., Cerambycidae). Entomol. Month. Mag. 122: 97-109.
Quicke, D. L. J. 1987. A new subfamily of Braconidae, the Vaepellinae, based on a new genus and species from Ghana (Insecta, Hymenoptera). Zool. Script. 16: 73-77.
Quicke, D. L. J. 1993. The polyphyletic origin of endoparasitism in the cyclostome lineages of Braconidae (Hymenoptera): a reassessment. Zool. Meded. 67: 159-177.
Quicke, D. L. J. and C. van Achterberg. 1990. Phylogeny of the subfamilies of the family Braconidae (Hymenoptera: Ichneumonoidea). Zool. Verhand. 258: 1-95.
Quicke, D. L. J., H. H. Basibuyk, M. G. Fitton and A. P. Rasnitsyn. 1999. Morphological, palaeontological and molecular aspects of ichneumonoid phylogeny (Hymenoptera, Insecta). Zool. Script. 28: 175-202.
Quicke, D. L. J. and R. Belshaw. 1999. Incongruence between morphological data sets: an example from the evolution of endoparasitism among parasitic wasps (Hymenoptera: Braconidae). Syst. Biol. 48: 436-454
Rasnitsyn, A. P. 1983. Ichneumonoidea (Hymenoptera) from the Lower Cretaceous of Mongolia. Contrib. Amer. Entomol. Inst. 20: 259-265.
Sanchis, A., A. LaTorre, F. Gonzalez-Candelas, and J. M. Michelena. 2000. An 18S rDNA based molecular phylogeny of Aphidiinae (Hymenoptera: Braconidae). Mol. Phylogen. Evol. 14: 180-194.
Sharkey, M. J. 1993. Family Braconidae. Pp. 362-395 in: Goulet, H. and J. T. Huber, eds., Hymenoptera of the World: An Identification Guide to Families. Agriculture Canada.
Sharkey, M. J. and D. B. Wahl. 1992. Cladistics of the Ichneumonoidea (Hymenoptera). J. Hymenopt. Res. 1: 15-24.
Shaw, M. R. and T. Huddleston. 1991. Classification and Biology of Braconid Wasps (Hymenoptera: Braconidae). Handbooks for the Identification of British Insects, Vol. 7, Part 11. Royal Entomological Society of London.
Shaw, S. R. 1995. Braconidae. Pp. 431-463 in: Hanson, P. E. and I. D. Gauld, eds. The Hymenoptera of Costa Rica, Oxford University Press.
Stoltz, D. B. 1986. Interactions between parasitoid-derived products and host insects: an overview. J. Ins. Physiol. 32: 347-350.
Stoltz, D. B. and S. B. Vinson. 1979. Viruses and parasitism in insects. Adv. Virus Res. 24: 125-171.
Stoltz, D. B. and J. B. Whitfield. 1992. Viruses and virus-like entities in the parasitic Hymenoptera. J. Hymenopt. Res. 1: 125-139.
Tobias, V. I. 1967. A review of the classification, phylogeny and evolution of the family Braconidae (Hymenoptera). Entomol. Rev. 46: 387-399.
Wharton, R. A. 1993a. Bionomics of the Braconidae. Ann. Rev. Entomol. 38: 121-143.
Wharton, R. A. 1993b. Review of the Hormiini (Hymenoptera: Braconidae) with a description of new taxa. J. Nat. Hist. 27: 107-171.
Wharton, R. A. 2001. Can braconid classification be restructured to facilitate portrayal of relationships? Pp. 143-153 in: Austin, A. D. and M. Dowton, eds., Hymenoptera: Evolution, Biodiversity and Biological Control. CSIRO Publishing, Collingwood, VIC.
Wharton, R. A., P. M. Marsh and M. J. Sharkey, eds. 1997. Manual of the New World genera of the family Braconidae (Hymenoptera). Int. Soc. Hymenopt. Spec. Pub. 1.
Wharton, R. A., P. M. Marsh, M. J. Sharkey and I Mercado, eds. 1998. Manual para los Generos de la Familia Braconidae (Hymenoptera) del Nuevo Mundo. Int. Soc. Hymenopt. Spec. Pub. 1. (Spanish edition of 1997 publication above).
Wharton, R. A., S. R. Shaw, M. J. Sharkey, D. B. Wahl, J. B. Woolley, J. B. Whitfield, P. M. Marsh and J. W. Johnson. 1992. Phylogeny of the subfamilies of the family Braconidae (Hymenoptera: Ichneumonoidea): a reassessment. Cladistics 8: 199-235.
Whitfield, J. B. 1990. Parasitoids, polydnaviruses and endosymbiosis. Parasitol. Today 6: 381-384.
Whitfield, J. B. 1992. The polyphyletic origin of endoparasitism in the cyclostome lineages of Braconidae (Hymenoptera). Syst. Entomol. 17: 273-286.
Whitfield, J. B. 1997. Molecular and morphological data suggest a single origin of the polydnaviruses among braconid wasps. Naturwiss. 84: 502-507.
Whitfield, J. B. 2002. Estimating the age of the polydnavirus/braconid wasp symbiosis. Proceedings of the National Academy of Sciences of the USA 99: 7508-7513.
Whitfield, J. B. and S. Asgari. 2003. Virus or not? phylogenetics of polydnaviruses and their wasp carriers. J. Ins. Physiol. 49: 397-405.
Whitfield,J. B., P. Mardulyn, A. D. Austin, and M. Dowton. 2002. Phylogenetic relationships among microgastrine braconid wasp genera based on data from the 16S, COI and 28S genes and morphology. Syst. Entomol. 27: 337-359.
Whitfield, J. B. and W. R. M. Mason. 1994. Mendesellinae, a new subfamily of braconid wasps (Hymenoptera, Braconidae) with a review of relationships within the microgastroid assemblage. Syst. Entomol. 19: 61-76.
Title Illustrations

| Scientific Name | Cardiochilinae |
|---|---|
| Specimen Condition | Dead Specimen |
| Sex | Female |
| Body Part | habitus |
| View | lateral |
| Copyright |
© 2004 Michael Sharkey

|
| Scientific Name | Doryctinae |
|---|---|
| Specimen Condition | Dead Specimen |
| Sex | Female |
| Life Cycle Stage | adult |
| Body Part | habitus |
| View | lateral |
| Copyright |
© 2004 Michael Sharkey

|
| Scientific Name | Homolobinae |
|---|---|
| Specimen Condition | Dead Specimen |
| Sex | Female |
| Body Part | habitus |
| View | lateral |
| Copyright |
© 2004 Michael Sharkey

|
About This Page
James B. Whitfield

University of Illinois Urbana, Illinois, USA
Won-Young Choi

National Institute of Biological Resources, Gyeongseo-Dong, Seo-gu, Incheon, South Korea

Centralamerican Institute for Biological Research and Conservation (CIBRC), Costa Rica
Josephine Rodriguez

University of Illinois, Urbana, Illinois, USA
Andrew R. Deans

Department of Entomology, NC State University
Correspondence regarding this page should be directed to James B. Whitfield at and Andrew R. Deans at
Page copyright © 2004 James B. Whitfield and Andrew R. Deans
 Page: Tree of Life
Braconidae.
Authored by
James B. Whitfield, Won-Young Choi, Alejandro A. Valerio, Josephine Rodriguez, and Andrew R. Deans.
The TEXT of this page is licensed under the
Creative Commons Attribution License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Braconidae.
Authored by
James B. Whitfield, Won-Young Choi, Alejandro A. Valerio, Josephine Rodriguez, and Andrew R. Deans.
The TEXT of this page is licensed under the
Creative Commons Attribution License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
- First online 10 June 2004
Citing this page:
Whitfield, James B., Won-Young Choi, Alejandro A. Valerio, Josephine Rodriguez, and Andrew R. Deans. 2004. Braconidae. Version 10 June 2004. http://tolweb.org/Braconidae/23447/2004.06.10 in The Tree of Life Web Project, http://tolweb.org/












 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site