Bryophyta
Mosses
Efrain De Luna, Angela E. Newton, and Brent D. Mishler


This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
Diversity of mosses has been classified in approximately 10,000 species, 700 genera, and about 110-120 families. This places the mosses as the third most diverse group of land plants, only after the angiosperms and ferns. Mosses are small plants requiring stereoscopes and compound microscopes for routine examination. The conspicuous green leafy shoots are the gametophytes, haploid organisms, on which the diploid embryo develops into a mature sporophyte (Figure 1). The sporophyte is chlorophyllose and photosynthetic only in early stages of development, and it is mostly dependent on the gametophyte. Moss colonies are a very important element in many ecosystems, from the tundra to the tropical rain forest, reducing soil erosion, capturing water and nutrients, providing shelter for microfauna, and nurseries for seedlings in succession or regeneration processes.
As a lineage, mosses are a historically crucial group in the understanding of the transition to life on land. The green leafy shoots (gametophytes) retain some features of the green algal ancestors (chlorophylls a and b, starch, sperm with two forward undulipodia), but the needle-like shoots that produce the spores (sporophytes) display key innovations for the life outside water, such as stomates, a simple strand of conductive cells [in an unbranched sporophyte], and airborne spores produced in a single apical capsule (sporangium). This is the simplest structural level among all land plants. The next organizational level is found in two fossil groups: Horneophythopsida and Aglaophyton (Rhynia) major, where the sporophyte is branched and produces several sporangia. The sporophyte shows the most complex structural organization in the tracheophytes.
Characteristics
There is no controversy that the mosses are monophyletic. Synapomorphies for the mosses are: i) leaves in the gametophyte; these green laminar organs are attached densely along the shoot. ii) multicellular rhizoids; these branched filaments composed of a series of multiple cells develop from the surface of the gametophyte axis at the point of contact with the substrate. iii) columnella; this is a cylinder of sterile cells located in the center of the capsule. (Mishler & Churchill, 1984). Other possible synapomorphies are features of male gamete [spermatozoid] ultrastructure (Mishler, Lewis & et al 1994).

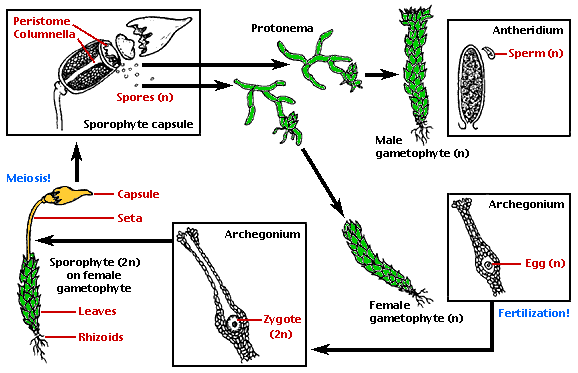
Figure 1. Life cycle of a dioicous moss.
Modified from original drawings by Ivy Livingstone. © 1997 BIODIDAC Modified from original drawings by Ivy Livingstone.
The life cycle of a moss alternates a conspicuous gametophyte generation and a dependent sporophyte generation. Some of the salient features of the different life cycle stages are outlined below.
Protonema. Spores germinate and produce a protonema. This is usually filamentous and branched, but in some groups it is tallose or massive. At several places in the protonema, apical cells differentiate and produce the foliose shoots.
Gametophore. An apical cell produces a stem and leaves spirally arranged. The stems produce branches in several combinations of monopodial and sympodial architectures. Leaves are sessile, unlobed, and often with a thickened midrib. Other appendages to the stem are multicellular rhizoids, axillary hairs, paraphyllia, pseudoparaphyllia, and various types of asexual propagules. The patterns of the leaf cell network and leaf cell papillae provide numerous characters for the systematic arrangement of genera and species.
Gametangia. Archegonia and antheridia are produced in groups, with paraphyses among them, and surrounded by perichaetial or perigonial leaves. Autoicous, synoicous, paroicous, heteroicous, and dioicous sexual conditions are found in many taxa.
Seta. The sporophyte consists of the foot, a seta and an apical sporangium. The foot is embedded within the apex of a stem or branch. Ultrastructural details of the transfer zone provide important similarities with other land plants. The seta is short or elongate. It has an internal conductive system that connects the foot and the capsule at both extremes.
Sporangium. A single sporangium or capsule develops distally from an unbranched sporophyte. The sporangium opens by an apical pore, longitudinal splits or most commonly by an operculum. The external cell layer (exothecium) often has stomata, especially in the neck. Underlaying the exothecium there are parenchymatose cells. Both concentric layers constitute the amphithecium (Figure 2A). Internally, the endothecium consists of a cylinder of sporogenous tissue, surrounding a columnella of sterile cells. Moss spores are unicellular, sometimes retaining tetrad marks.

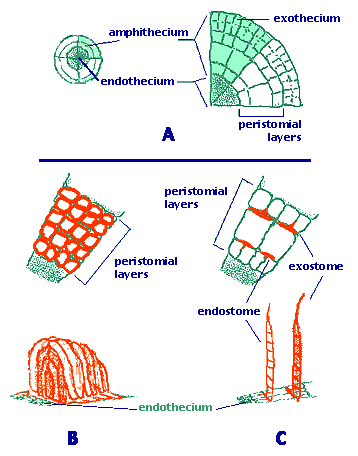
Figure 2. The two basic peristome types of mosses.
A. Cross section of an embryonic sporophyte capsule.
B. Nematodontous peristome (e.g., Polytrichum).
C. Arthrodontous peristome (e.g., Funaria or Bryum).
Drawings copyright © 2000 Efrain De Luna
Peristome. In the majority of mosses, the apex of the capsule (operculum) falls off at maturity and reveals a structure called the peristome (Figure 1). This is a ring of narrow triangular segments surrounding the mouth of the capsule. Changes in moisture conditions cause movements of the peristome and facilitate the dispersion of spores in favorable dry conditions. Two basic types of peristomes are found in mosses: arthrodontous and nematodontous. In arthrodontous peristomes, at the level of the capsule mouth and above, three innermost rings of cells of the amphithecium are involved in the formation of teeth in most taxa. These three concentric rows are known as the "outer", "primary" and "inner" peristomial layers (OPL, PPL, IPL). Most peristomate mosses have the arthrodontous type, in which each tooth is composed of periclinal (tangential) cell wall remnants between two of the three concentric peristomial cell layers (Figure 2C). If the teeth are formed by the tangential walls between the OPL and PPL, the row of teeth is collectively known as the exostome. In the second case, the cell wall remnants are located between the cell rings of the PPL and IPL, therefore the row of segments is known as endostome. A second fundamental type of peristome is nematodontous, structured by narrow columns of entire cell wall remnants. Each tooth consists of agglomerated cylinders formed by the periclinal and anticlinal thickened cell walls (Figure 2B). As in arthrodontous peristomes, peristomial cells derive from the innermost amphithecium, but multiple concentric peristomial layers (four to seven) contribute to the formation of a nematodontous tooth.
Discussion of Phylogenetic Relationships
Early proposals of phylogenetic relationships within the mosses (e.g., Vitt 1984) interpreted three fundamental levels of structural organization, largely based on the morphology of the capsules. In the most basal lineage, the Sphagnopsida, capsules open through an apical pore. In the Andreaeopsida, a very small intermediate group, capsules open through longitudinal valves. In the most derived and diverse lineage, the "true mosses" (commonly classified as the Bryopsida), the capsules are operculate and differentiate a peristome. Characters from the exostome and endostome provided a basis for classification of major groups at the ordinal level and above.
Modern explicit hypotheses for moss relationships are based on several cladistic analyses based on morphological, ultrastructural, and molecular data. A recent issue of the journal "The Bryologist" (Vol 103, issue 2, 2000) includes a number of new phylogenetic analyses that incorporate exemplars from nearly all families of mosses. Each paper was a major collaborative effort by researchers from many countries who brought together sequence data from rbcL, rps4, trnL-F, and 18S rRNA. Newton et al. (2000, Fig. 3E) presented a cladistic analysis of all orders of mosses based on morphological characters and four DNA sequence data sets for 33 exemplar taxa and ten outgroups. Results include the monophyly of the mosses, the inclusion of Takakia as a moss sister to Sphagnum, and the monophyly of the peristomate mosses. In combination, these recent higher level analyses provide the first cladistic framework for the major lineages of mosses.
Five main lineages are currently recognized (Mishler & Churchill 1984, Newton et al., 2000), although their taxonomic ranking as classes or subclasses is still controversial. In most cases, higher-level classification of the mosses is not fully settled because there are different names used for the same major clades. Here we use names for these five lineages considered at the rank of class. A basal split divides the Sphagnopsida (1) sister to a large clade in which the Andreaeopsida (2) is the most basal lineage (Figure 3A, B). Peristomate mosses include three main lineages: Polytrichopsida (3) with nematodontous peristomes are basal to a clade of arthrodontous mosses that includes the Tetraphidopsida (4) and Bryopsida (5) (Figure 3B).

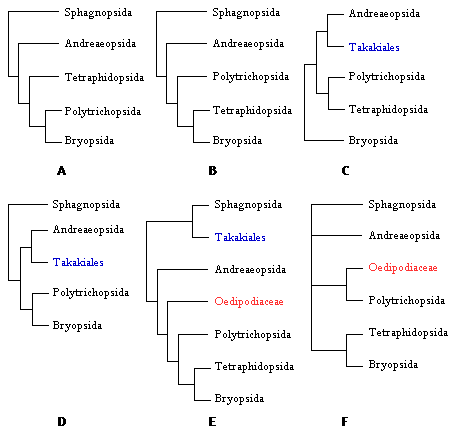
Figure 3. Alternative placements of Polytrichopsida, Tetraphidopsida, Takakiales, and Oedipodiaceae. A. Tree from Mishler & Churchill (1984, p. 412). B. Tree from Beckert et al. (1999, p. 185). C. Tree from Hedderson et al. (1996, p. 217). D. Tree from Mishler et al. (1994, p. 466). E. Tree from Newton et al. (2000, p. 195). F. Tree from Hyvonen et al. (1998, p. 498).
Early schemes of relationships within mosses have not changed substantially, but they have been expanded to accommodate a few groups now recognized at higher taxonomic rankings. Ambuchania, a separate genus closely related to Sphagnum, is now classified in its own order and placed as sister to the Sphagnales (Shaw 2000). Andreaeobryum, a genus related to Andreaea, is also classified in its own order and placed as sister to the Andreaeales (Newton et al. 2000).
Ideas about the relationships within the peristomate clade have oscillated between two views. In some systems, the Polytrichopsida were considered as the most advanced because of the complexity of the gametophyte (Brotherus 1925). In contrast, other arrangements interpreted this group as the most ancestral due to the simplicity of the nematodontous peristome (Vitt 1984). Modern cladistic analyses corroborated the basal position of the nematodontous mosses (Polytrichum) relative to the more recent origin of the diplolepidous mosses (Bryum). However, the phylogenetic positions of certain groups are still controversial. The Tetraphidopsida, for example, has been placed as sister to the Bryopsida or sister to the Polytrichopsida and Bryopsida together (Figure 3A, B, C). Another example of controversial phylogenetic position is the Oedipodiaceae. This family is alternatively placed as sister to the large clade of peristomate mosses (Polytrichopsida, Tetraphidopsida and Bryopsida) or as sister to just the Polytrichales (Figure 3E, F).
The fascinating bryophyte Takakia deserves a special note. Initially it was interpreted as a liverwort, and until recently it was classified in the Calobryales (Schofield 1985). After the discovery of plants with sporophytes, now it is undoubtedly classified as a moss. This placement within the mosses was confirmed with recent cladistic analyses based on morphology and several genes, although it remains uncertain if it should be sister to Andreaeaopsida or Sphagnopsida (Figs. 3C, D, E).
Several factors have made possible such new insight on higher level relationships within mosses. New empirical data have accumulated on morphology, anatomy, ontogeny, ultrastructure, and DNA sequences. Although most of the recent studies at higher levels are based on molecular data, the most important element of the recent progress has been the use of cladistic methodology for the interpretation of such types of evidence. A formal framework of higher-level moss phylogeny is now available, which replaces former intuitive estimates of relationships founded largely on concepts of overall similarity and ad hoc evolutionary scenarios.
References
Beckert, S., S. Steinhauser, H. Muhle and V. Knoop. 1999. A molecular phylogeny of bryophytes based on nucleotide sequences of the mitochondrial nad5 gene. Pl. Syst. Evol. 218:179-192.
Bremer, K. 1985. Summary of green plant phylogeny and classification. Cladistics 1:369-385.
Capesius, I. and M. Stech. 1997. Molecular relationships within mosses based on 18S rRNA gene sequences. Nova Hedwigia 64:525-533.
Brotherus, V. F. 1925. Musci (Laubmoose), p. iii, In: A. Engler & K. Plant (eds). Die Natürlichen Pflanzen-familien. Vol 10. W. Engelmann, Leipzig.
Duff, R. J. and D. L. Nickrent. 1999. Phylogenetic relationships of land plants using mitochondrial small-subunit rDNA sequences. American Journal of Botany 86:372-386.
Garbary, D. G., K. Renzaglia and J. G. Duckett. 1993. The phylogeny of land plants: a cladistic analysis based on male gametogenesis. Pl. Syst. Evol. 188:237-269.
Hedderson, T. A., R. L. Chapman and W. L. Rootes. 1996. Phylogenetic relationships of bryophytes inferred from nuclear encoded rRNA gene sequences. Pl. Syst. Evol. 200:213-224.
Hyvönen, J., T. A. Hedderson, G. L. Smith-Merrill, J. G. Gibbings and S. Koskinen. 1998. On phylogeny of the Polytrichales. The Bryologist 101:489-504.
Kenrick, P. and P. R. Crane. 1991. Water-conducting cells in early fossil land plants: implications for the early evolution of tracheophytes. Botanical Gazette 152:335-356.
Kenrick, P. and P. R. Crane. 1997. The origin and early diversification of land plants: a cladistic study. Smithsonian Institute Press, Washington, DC.
Margulis, L. and K. V. Schwartz. 1996. Five Kingdoms: An illustrated guide to the phyla of life on Earth. W. H. Freeman an Co. New York.
Mishler, B. D. and S. P. Churchill. 1984. A cladistic approach to the phylogeny of the `bryophytes'. Brittonia 36:406-424.
Mishler, B. D., P. Thrall, J. S. Hopple Jr., E. De Luna and R. Vilgalys. 1992. A molecular approach to the phylogeny of bryophytes: cladistic analysis of chloroplast-encoded 16S and 23S ribosomal RNA genes. The Bryologist 95:172-180.
Mishler, B. D., L. A. Lewis, M. A. Buchheim, K. S. Renzaglia, D. J. Garbary, C. F. Delwiche, F. W. Zechman, T. S. Kantz and R. L. Chapman. 1994. Phylogenetic relationships of the "green algae" and "bryophytes". Annals of the Missouri Botanical Garden 81:451-483.
Newton, A. E., C. J. Cox, J. G. Duckett, J. A. Wheeler, B. Goffinet, T. A. Hedderson and B. D. Mishler. 2000. Evolution of the major moss lineages: Phylogenetic analyses based on multiple gene sequences and morphology. The Bryologist 103:187-211.
Schofield, W. B. 1985. Introduction to Bryology. Macmillan Publishing Company, New York.
Shaw, A. J. 2000. Phylogeny of the Sphagnopsida based on chloroplast and nuclear DNA sequences. The Bryologist 103: 277-306.
Vitt, D. H. 1984. Classification of the Bryopsida. In: R. M. Schuster (ed). New Manual of Bryology, vol 2: 696-759. The Hattori Botanical Laboratory, Nichinan, Japan.
Information on the Internet
- Bryology at Missouri Botanical Garden.
- Phylum Bryophyta: The Mosses. Land Plants Online, Southern Illinois University at Carbondale.
- Introduction to the Bryophyta. UCMP Berkeley.
- Bryophytes. C. M. Sean Carrington, University of the West Indies.
- Bryophyta. Michael Knee, Ohio State University.
- Mosses and Liverworts (Bryophyta). Kimball's Biology Pages.
- Mosses. Connecticut River Homepage.
- Mosses and Allies. Natural Perspective.
- Bryologie am Botanischen Institut der Universität Bonn.
- Briología en el Instituto de Ecología AC, Xalapa, Ver. México.
- La page des Bryophytes. Les Pages Nature, Jean Dexheimer.
- IUCN Species Survival Commission Bryophyte Specialist Group.
- Bryophyta (Mosses and Liverworts). BioImages: The Virtual Field-Guide (UK).
- Felix Schumm's Moss Gallery.
Societies
- Bryological Societies and Working Groups.
- International Association of Bryologists.
- American Bryological and Lichenological Society Home Page.
- Sociedad Latinoamericana de Briologia.
- British Bryological Society.
- British Bryological Society: Tropical Bryology Group.
- Dutch Bryological and Lichenological Society.
Floras and Checklists
-
North America
- Bryophyte Flora of North America.
- New York Botanical Garden: Catalog of North American Bryophytes.
- Moss of New England.
- Checklist of the Mosses of the Interior Highlands of North America in Arkansas, Ilinois, Missouri and Oklahoma.
- New Jersey Mosses.
- Checklist of the Mosses of North Carolina.
- Wisconsin Bryophytes.
-
Central and South America
- Moss Flora of Central America.
- Moss Flora of Chile Home Page.
-
Europe
- Mosses of the Former USSR.
- List of Estonian Bryophytes.
- British and Irish Bryophytes and Their Distribution.
- Mosses and Liverworts in Wales.
- Checklist of Danish Mosses.
- Checklist of Mosses in Stuttgart.
Title Illustrations

| Copyright |
© 2000 Efrain De Luna

|
|---|
| Copyright |
© 2000 Efrain De Luna

|
|---|
| Copyright |
© 2000 Efrain De Luna

|
|---|
About This Page
Efrain De Luna

Instituto de Ecologia AC., Xalapa, Veracruz, Mexico
Angela E. Newton

The Natural History Museum London, UK
Brent D. Mishler

University of California, Berkeley, California, USA
Correspondence regarding this page should be directed to Efrain De Luna at
Page copyright © 2003 Efrain De Luna
 Page: Tree of Life
Bryophyta. Mosses.
Authored by
Efrain De Luna, Angela E. Newton, and Brent D. Mishler.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Bryophyta. Mosses.
Authored by
Efrain De Luna, Angela E. Newton, and Brent D. Mishler.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
- First online 25 March 2003
Citing this page:
De Luna, Efrain, Angela E. Newton, and Brent D. Mishler. 2003. Bryophyta. Mosses. Version 25 March 2003. http://tolweb.org/Bryophyta/20599/2003.03.25 in The Tree of Life Web Project, http://tolweb.org/












 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site