Coelopidae
Kelp Flies
Keith Bayless


This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
Coelopidae Hendel is a small family of locally abundant flies with a nearly cosmopolitan distribution. The larvae of all 29 described species in 13 genera are important decomposers in beach ecosystems. Most consume stranded decaying brown algae (Phaeophyceae), particularly kelp (Laminariales), meriting the common name kelp flies. These flies are usually in the wrack zone of shores and are rarely found inland. Kelp flies are highly adapted to this lifestyle, and most species are obligatorily dependent on decomposing kelp. Coelopidae larvae (Figs. 1, 2) can survive for up to 6 days in aerated water, which helps them endure the vicissitudes of intertidal life (McAlpine 1991). A ring of unique hydrofuge hairs surrounding the larval spiracle aids respiration in submerged larvae (fig. 3).

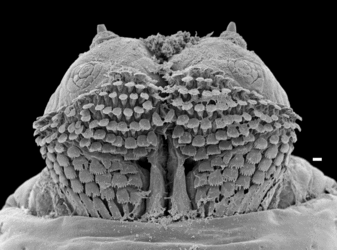
Figure 1. Pseudocephalon of Lopa convexa larva. Scale bar = 10 μm. © Rudolf Meier

Figure 2. Pseudocephalon of Chaetocoelopa sydneyensis larva. © Rudolf Meier
A few Australian species are also found in stranded seagrass (Zostera sp.). One species has been recorded from beached carrion. A few adults have been recorded at flowers. Stigmatomycetes fungus attack adults at fairly high rates (18% in McAlpine 1991), however, curiously, adult flies with fungal fruiting bodies survive and behave normally. A few species found on isolated islands such as Campbell Island (New Zealand) are brachypterous and unable to fly. More information about biology and habitats can be found in McAlpine (1991). The taxonomy and distribution of this family will be treated in depth in an upcoming checklist (Mathis and McAlpine, in press).
Coelopidae are most common in cool temperate climates. They are most diverse in Australia and New Zealand. There are no species endemic to the New World tropics. The unpredictable nature of kelp availability can lead to vast population explosions of coelopid flies when decaying kelp is abundant. During such times, these flies can become a nuisance to people and livestock, but they serve an essential role in the decomposition of beached kelp. Some species can be reared in laboratory settings.
Coelopid flies tend to be highly variable in size; some Coelopa frigida (Fabricius) can be three times larger than others. This is due partly to the availability and quality of larval food. A complex chromosomal inversion system is also involved in regulating body size in some species of Coelopa. This genetic oddity is a factor in the use of Coelopa frigida, C. nebularum Aldrich, and several other species as models in the study of speciation (e.g. Laamanen et al 2003), ecology (e.g. Hodge & Arthur 1997), and sexual selection (e.g. Crean et al 2000).
Characteristics
Coelopids are generally flattened and setose. They range from 2.5 to 16 mm in length. Coelopidae is a well-supported group using morphological characters. Tergites 7 and 8 are distinct in males, a rare and conjecturally basal condition in Schizophora. McAlpine (1991) posited that this condition is a vestige of the ancestral groundplan Schizophoran fly. Below is a list of the putative groundplan characters of the family (adapted from McAlpine 1991 and Meier and Wiegmann 2002):
- arrangement of peristigmatic tufts of setae of larvae in a ring around the spiracular plate (autapomorphy) (fig. 3);
 Click on an image to view larger version & data in a new window
Click on an image to view larger version & data in a new window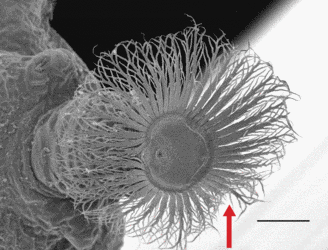
Figure 3. Larval spiracle of Amma blancheae. Arrow indicates tufts of setae. Scale bar = 100 μm. © Rudolf Meier
- larval habitat in decaying kelp (autapomorphy);
- postocellar setae convergent;
- antennae decumbent;
- prominence on parafacial ridge;
- distalmost seta of dorsal transverse series on scape enlarged, mesoclinate;
- scutum bearing distinct, medial series of setulae anteriorly;
- posterior portion of katepisternal suture shallowly projected dorsally, forming an arc above katepisternal setae;
- 1 or 2 anteroclinate to anterodorsoclinate katepisternal setae;
- thumbnail-like process on male protarsus (fig. 4);
 Click on an image to view larger version & data in a new window
Click on an image to view larger version & data in a new window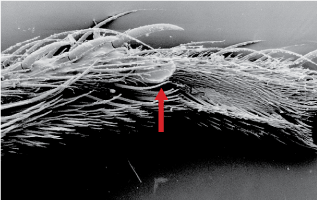
Figure 4. Ventral view of male protarsus. Arrow indicates thumbnail-like process. © Rudolf Meier
- apical tarsomeres broadly subtriangular with setiferous protuberances on apical margin;
- sternite 1 very short or vestigial.
Discussion of Phylogenetic Relationships
The most recent and comprehensive phylogenetic hypothesis of Coelopidae was that of Meier & Wiegmann (2002). Coelopidae are acalyptrate flies in the Sciomyzoidea (Cyclorrhapha). Within the Sciomyzoidea, the closest relative to Coelopidae has yet to identified. J. F. McAlpine and Woods (1989) considered the kelp flies to be the sister group to the remaining Sciomyzoidea. D. K. McAlpine (1991) noted several shared characters with Helcomyzidae, such as the parafacial prominence and thumbnail-like process on the male protarsus (Fig. 4). Meier and Weigmann (2002) posited that Coelopidae is sister to a clade consisting of Helcomyzidae, (Helosciomyzidae, Dryomyzidae).
The large number of genera in this species-poor group is due to significant morphological differentiation between the members of this family. Coelopidae is comprised of 2 subfamilies, the monospecific Lopinae consisting of Lopa convexa McAlpine (Fig. 1), and the Coelopinae. Under some weighting schemes in Meier & Wiegmann 2002, Lopa convexa was placed outside of Coelopidae.
McAlpine (1991) described 4 tribes in Coelopinae, the Coelopellini (Coelopella Malloch, Rhis McAlpine, This McAlpine), Ammini (Amma McAlpine, Baeopterus Lamb, Icaridion Lamb), Coelopini (Coelopa Meigen), and Glumini (Chaetocoelopa Malloch (Fig. 2), Coelopina Malloch, Dasycoelopa Malloch, Gluma McAlpine, Malacomyia Haliday). Ammini and Coelopellini are not supported in the most comprehensive phylogeny using morphological and molecular data (Meier & Wiegmann 2002). The genera included in Ammini and Coelopellini form a clade in Meier & Wiegmann (2002), as suggested by McAlpine 1991, but the tribes are not supported as monophyletic. There was strong support for Coelopella popeae being sister to Rhis whitleyi instead of the other Coelopella species in Meier & Wiegmann (2002). There is one undescribed New Zealand species/genus closely related to Baeopterus and Icaridion (nov. spec. in Meier & Wiegmann 2002).
Glumini is supported as monophyletic in Meier & Wiegmann (2002), but the monophyly of Coelopa remains in doubt as is validity of the two Coelopa subgenera (Fucomyia Haliday, Neocoelopa Malloch; McAlpine 1991, Mathis & McAlpine in press). Coelopa aequatorialis, C. dasypoda, C. orientalis, and C. stejnegeri were not included in Meier & Wiegmann (2002); they are placed in Coelopa incertae sedis. Coelopina is not included in Meier & Wiegmann (2002). It is morphologically very similar to Chaetocoelopa (McAlpine 1991), so it is presented here as Glumini incertae sedis. There was weak support for a sister group relationship between Dasycoelopa australis and Chaetocoelopa littoralis in Meier & Wiegmann (2002), so the monophyly of Chaetocoelopa is unclear.
R. Meier is currently working on a more comprehensive phylogeny (R. Meier, pers. comm.). Keys to the subfamilies, tribes, genera, and Australasian species are available in McAlpine (1991). Vockeroth (1987) and McAlpine (1998) address the Nearctic and Palearctic Coelopidae, respectively. The taxonomy and distribution of this family will be treated in depth in an upcoming checklist (Mathis and McAlpine, in press).
References
Crean, C. S., D. W. Dunn, T. H. Dat, and A. S. Gilburn, 2000. Female mate choice for large males in several species of seaweed fly (Diptera: Coelopidae). Anim. Behavior. 59:121-126.
Hodge, S., and W. Arthur. 1997. Asymmetrical interactions between species of seaweed fly. J. Anim. Ecol. 66:743-754
Laamanen, T. R., F. T. Petersen, and R. Meier, 2003. Kelp flies and species concepts – the case of Coelopa frigida (Fabricius, 1805) and C. nebularum Aldrich 1929 (Diptera: Coelopidae). J. Zool. Syst. Evol. Research 41:127-136.
Mathis, W. N., and D. K. McAlpine. In press. A Catalog and Conspectus on the Family Coelopidae (Diptera: Schizophora).
McAlpine, D. K. 1998. Chapter 31. Family Coelopidae. Manual Palaearct. Dipt. 3: 335-340.
McAlpine, D. K. 1991. Review of the Australian kelp flies (Diptera: Coelopidae). Syst. Entomology. 16:29-84.
McAlpine, J. F. and D. M. Wood (eds.). 1989. Manual of Nearct. Dipt. 3. Research Branch, Agriculture Canada, Monograph 32.
Meier, R., and B. M. Wiegmann. 2002. A phylogenetic analysis of Coelopidae (Diptera) based on morphological and DNA sequence data. Mol. Phylogenet. Evol. 25:393-407.
Vockeroth, J. R. 1987. 82. Coelopidae. Manual of Nearct. Dipt. 2:919-922
Title Illustrations

| Scientific Name | Schizophora |
|---|---|
| Location | Western Australia |
| Specimen Condition | Live Specimen |
| Identified By | Keith Bayless |
| Life Cycle Stage | Adult |
| Copyright |
© Steve Marshall

|
About This Page
Keith Bayless

North Carolina State University, Raleigh, North Carolina, USA
Correspondence regarding this page should be directed to Keith Bayless at
Page copyright © 2007 Keith Bayless
All Rights Reserved.
- First online 13 September 2007
- Content changed 20 December 2007
Citing this page:
Bayless, Keith. 2007. Coelopidae. Kelp Flies. Version 20 December 2007. http://tolweb.org/Coelopidae/10652/2007.12.20 in The Tree of Life Web Project, http://tolweb.org/







 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site