Core Eudicots
Core Tricolpates
Doug Soltis, Pam Soltis, and Christine Edwards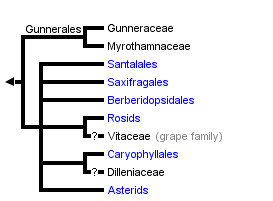


This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
The core eudicots are an extremely large, diverse assemblage of flowering plants, with an enormous range of variation in habit, morphology, chemistry, geographic distributions, and other attributes. Based on analyses of morphology, classical systematists did not previously recognize the core eudicot group. Instead, the circumscription of the core eudicots as a clade was based on the very strong support obtained in analyses of DNA data sets (e.g., Hoot et al., 1999; Savolainen et al., 2000a; P. Soltis et al., 1999; D. Soltis et al., 2000).
Characteristics
Although the core eudicot group was not originally identified based on morphological evidence, subsequent research has identified several key events that correspond fairly closely to the origin of the core eudicots, including the evolution of flowers organized in a predictable manner with a stable number of parts (e.g., flowers with parts in fives or multiples of five, a clear differentiation of sepals and petals, twice the number of stamens as petals, and a gynoecium of three to five typically fused (at least partially) carpels; Judd et al. 2002), production of ellagic and gallic acids, and perhaps the duplication of several floral organ identity genes including homologs of the Arabidopsis genes, Apetala3 (Kramer et al., 1998) and Apetala1 (Litt and Irish, 2003; reviewed in D. Soltis et al., 2005).
Discussion of Phylogenetic Relationships
The strongly supported core eudicot clade comprises seven generally strongly supported subgroups (D. Soltis et al., 2000, 2003; APG II, 2003): 1) Gunnerales (Myrothamnaceae and Gunneraceae; 2) "Berberidopsidales" (an order that was not recognized by APG II and comprising Berberidopsidaceae and Aextoxicaceae); 3) Saxifragales; 4) Santalales; 5) rosids; 6) Dilleniaceae/Caryophyllales; and 7) asterids. Recent studies identified Gunnerales as the sister to all other core eudicots (D. Soltis et al., 2003; Hilu et al., 2003); however, relationships among the remaining lineages are largely unresolved. The circumscription and general phylogenetic relationships of each of these major groups are discussed below. For further discussion of these phylogenetic relationships, see D. Soltis et al. (2005) and subsequent Tree of Life web pages.
Gunnerales
Gunnerales comprise two small families, Gunneraceae (Gunnera with approximately 40 species) and Myrothamnaceae (Myrothamnus with two species), or Gunneraceae s.l. sensu APG II (2003). This clade was identified based on high molecular support for this relationship; it had not previously been suggested on the basis of morphology because the two genera differ substantially morphologically. Gunneraceae have a dimerous perianth (e.g., the calyx and corolla each with two parts, or a multiple of two parts; Drinnan et al., 1994), as do many of the basal eudicot lineages; dimery is found in Buxaceae, Trochodendraceae, and Proteaceae (but perhaps not the Platanus lineage) and is common and perhaps ancestral in Ranunculales (van Tieghem, 1897; Drinnan et al., 1994; Douglas and Tucker, 1996). The placement of Gunnerales as sister to the rest of the core eudicots implies that the pentamerous perianth typical of most core eudicots was derived from dimerous ancestors (Ronse DeCraene et al., 2003; D. Soltis et al., 2003).



Two views of Gunnera sp. (Gunneraceae) including a view of an entire plant and the leaf of one plant (photos © Douglas Soltis).
"Berberidopsidales"
Like Gunnerales, "Berberidopsidales" comprise two small and morphologically disparate families: Berberidopsidaceae (Berberidopsis and Streptothamnus; the latter is sometimes included in Berberidopsis) and Aextoxicaceae (Aextoxicon, one species). Although this clade has not been recognized at the ordinal level by APG (hence the quotation marks), it is strongly supported by molecular data and is isolated from all other clades. Furthermore, both families have encyclocytic stomata, a rare character and an apparent synapomorphy for this clade (D. Soltis et al., 2005). Hence, future revision of the APG classification will likely recognize Berberidopsidales (see Soltis et al., 2005). With four genes, Berberidopsidaceae plus Aextoxicaceae ("Berberidopsidales") received weak support (54%) as sister to the remaining core eudicots following Gunnerales. Thus, at this point the placement of Berberidopsidales should be considered uncertain.
Saxifragales
Saxifragales are a morphologically eclectic clade of annual and perennial herbs, succulents, aquatics (rarely), shrubs, vines, and large trees. The composition of Saxifragales based on molecular analyses was not suggested in any traditional treatment (Morgan and Soltis, 1993; Fishbein et al., 2001); indeed, members of this clade were classified in three of Cronquist's (1981) six subclasses of dicots (see also Takhtajan, 1997). Possible synapomorphies for this clade include a partially fused gynoecium with two carpels, a hypanthium, and glandular leaf teeth (Judd et al., 2002); also, aspects of leaf venation and wood anatomy are similar in the woody members of the clade. The best known of the 13 families in this clade are Saxifragaceae, Crassulaceae, Grossulariaceae, Paeoniaceae, and Hamamelidaceae. Molecular studies continue to reveal new, unexpected members of this clade, such as Aphanopetalum (Fishbein et al., 2001) and Peridiscaceae (Davis and Chase, 2004), a family placed in Malpighiales in APG II (2003).
The monophyly of Saxifragales is strongly supported, but the position of this clade relative to other core eudicots remains uncertain. Some analyses have placed it as sister to the rosids, although with weak support (e.g., D. Soltis et al., 2000). The simple, pentamerous flowers of many Saxifragaceae have long been thought to indicate a relationship with Rosaceae and other rosids, but whether these floral features are synapomorphies for Saxifragales + rosids or symplesiomorphies (i.e., shared ancestral features) is unclear. Additional research is needed to resolve the relationship of Saxifragales within the core eudicots (see D. Soltis et al., 2005).




Representatives of the Saxifragales, including Boykinia major (Saxifragaceae; photo © Douglas Soltis), Fothergilla gardenii (Hamamelidaceae; photo © Walter Judd), and Crassula argentea (Crassulaceae; photo © Scott Zona).
Santalales
The seven families of Santalales are a strongly supported clade of core eudicots and are united by both molecular characters and morphological features associated with the parasitic habit found in most members. However, relationships of Santalales to other core eudicots are not clear, although they often appear related to the asterids and Caryophyllales + Dilleniaceae in at least some shortest trees (D. Soltis et al., 2000). Furthermore, evolutionary relationships within Santalales have not yet been fully resolved, which may be explained in part by apparently rapid rates of molecular evolution in all three plant genomes (e.g., Nickrent and Starr, 1994; Nickrent et al., 1998). However, DNA evidence has revealed that several of the currently recognized families probably do not represent single evolutionary lineages (Nickret and Malecot, 2001; APG II, 2003; D. Soltis et al., 2005).




Representatives of the Santalales, including a Dendropthora opuntioides with fruits (Santalaceae; photo © Walter Judd), a branch of Phorodendron leucarpum (Viscaceae; photo © Paul Corogin), and the visible red fruits of Viscum minimum, which is growing as a parasite on Euphorbia polygona (Viscaceae; photo © Scott Zona).
Caryophyllales + Dilleniaceae
The core of Caryophyllales sensu APG II (2003) was considered a closely related group of families as long ago as the mid-nineteenth century (e.g., Braun, 1864; Eichler, 1876) and was formally recognized as the Centrospermae by Harms (1934) based on morphological and embryological characters. Chemical data (presence of betalains) altered the concept of core Caryophyllales (Clement and Mabry 1996). Cladistic analyses of morphological and chemical data defined the group and provided hypotheses of relationships among the families (Rodman et al., 1993; reviewed in D. Soltis et al., 2005). Recent molecular studies have identified a larger clade (Caryophyllales sensu APG II) that includes the Caryophyllidae of Cronquist (1981; i.e., Caryophyllales, Polygonales, and Plumbaginales) plus a number of families previously considered distantly related to Caryophyllales, including the carnivorous sundews and Venus flytrap (Droseraceae) and Old World pitcher plants (Nepenthaceae). Within Caryophyllales sensu lato, there are two large clades, core and non-core Caryophyllales (Cuénoud et al., 2002), that correspond to Caryophyllales and Polygonales sensu Judd et al. (2002). Naming the larger clade "Caryophyllales" has resulted in some confusion, and not all investigators follow the APG system. For example, Judd et al. (2002) continue to use Caryophyllales in the narrow sense and refer to the larger clade as the caryophyllids. This system has advantages and perhaps should be adopted in future versions of the APG system (D. Soltis et al., 2005).
Relationships of Caryophyllales to other core eudicots are not clear, although Dilleniaceae are sister to Caryophyllales in most analyses, although generally with low support (e.g., Chase and Albert, 1998; D. Soltis et al., 2000). Furthermore, some evolutionary trees have indicated a possible relationship of Caryophyllales with the asterids and Santalales.




Representatives of the Caryophyllales, including the flower of the cactus, Hylocereus undatus (Cactaceae; photo © Walter Judd), a branch of Cocoloba uvifera with fruits (Polygonaceae; photo © Walter Judd), and the ornamental, Celosia argentia, in flower (Amaranthaceae; photo © Walter Judd).
Rosids + Vitaceae
The rosid clade as defined by molecular data is broader than the traditional subclass Rosidae (Cronquist, 1981; Takhtajan, 1980, 1997) and encompasses many families formerly classified in the polyphyletic subclasses Magnoliidae, Dilleniidae, and Hamamelidae. The rosids comprise 140 families and close to one-third of all angiosperm species (D. Soltis et al., 2005). Clear synapomorphies for the rosids have not been identified, although most rosids share several morphological and anatomical features, such as nuclear endosperm development, reticulate pollen exine, generally simple perforations of vessel end-walls, alternate intervessel pitting, mucilaginous leaf epidermis, and two or more whorls of stamens, plus ellagic acid (Hufford, 1992; Nandi et al., 1998; reviewed in D. Soltis et al., 2005).




Representatives of the rosid clade, including the flowers of Sorbus aucuparia (Rosaceae: Rosales: eurosid I; photo © Walter Judd), the flower of the passion flower, Passiflora coerulea var. cinncinata (Passifloraceae: Malpighiales: eurosids I; photo © Sangtae Kim), and the flowers and fruits of Clusia rosea (Clusiaceae: Malpighiales: eurosids I; photo © Walter Judd).
Relationships within the rosid clade are not clearly resolved. Vitaceae may be sister to all other rosids, but this relationship is not strongly supported (Savolainen et al., 2000a, b; reviewed in D. Soltis et al., 2000). Saxifragales may be sister to the Vitaceae + rosids clade, but this relationship is also not strongly supported (D. Soltis et al., 2000, 2005). Two large subclades of rosids, eurosids I (fabids) and II (malvids), have been identified through molecular analyses (e.g., Chase et al., 1993; Savolainen et al., 2000a, b; D. Soltis et al., 2000). The eurosid I clade comprises Celastrales, Cucurbitales, Fabales, Fagales, Zygophyllales, Malpighiales, Oxalidales, and Rosales. Of these, Cucurbitales, Fabales, Fagales, and Rosales form the "nitrogen-fixing clade," a clade that contains all angiosperms known to have symbiotic relationships with nodulating nitrogen-fixing bacteria (see D. Soltis et al., 1995, 1997, 2000). The smaller eurosid II clade is composed of Brassicales, Malvales, Sapindales, and Tapisciaceae. In addition to the large eurosid I and II clades, additional smaller clades have been recognized (Crossosomatales, Myrtales, Geraniales, and Picramniaceae), but their relationships to each other and to eurosids I and II are not clear. Furthermore, relationships within eurosids I and II are not fully resolved, and much additional work is needed to reconstruct relationships within the rosid clade. In fact, the rosids represent the largest remaining problematic group of angiosperms. For a more detailed discussion of the rosids, see D. Soltis et al. (2005).




More representatives of the rosid clade, including the flowers of Euphorbia pulcherrima (Euphorbiaceae: Malpighiales: eurosids I; photo © Walter Judd), the flowers of the legume, Albizzia julibrisin (Fabaceae: Fabales: eurosids I; photo © Walter Judd), and the flower of Meriania brevipedunculata (Melastomataceae: Myrtales; photo © Walter Judd).
Asterids
Like rosids, asterids are a large clade, encompassing nearly one-third of all angiosperm species (80,000 species) classified in 114 families (Albach et al., 2001a; D. Soltis et al., 2005). However, unlike the rosids, a group of families corresponding closely to the asterid clade has been recognized on morphological grounds for over 200 years (de Jussieu, 1789; Reichenbach, 1828; Warming, 1879), and several morphological and chemical features appear to unite all or most asterids. Most notable are iridoid chemical compounds (e.g., Jensen, 1992), corollas with fused petals, unitegmic and tenuinucellate ovules, and cellular endosperm development; however, it is still unclear which of these features are actually synapomorphies for asterids (cf. Albach et al., 2001b; Judd et al., 2002). The asterid clade is broader than the Asteridae of recent classifications (e.g., Cronquist, 1981; Takhtajan, 1980, 1997) and includes also members of the polyphyletic subclasses Hamamelidae, Dilleniidae, and Rosidae (Olmstead et al., 1992, 1993, 2000; Chase et al., 1993; D. Soltis et al., 1997, 2000; P. Soltis et al., 1999; Savolainen et al., 2000a, b).
Many relationships within asterids were resolved by angiosperm-wide molecular phylogenetic analyses, but asterids have since been analyzed in greater detail with more extensive taxon sampling and data from four (Albach et al., 2001a) and six (B. Bremer et al., 2002) loci. These studies confirmed earlier results indicating four major clades within asterids: Cornales and Ericales are subsequent sisters to all other asterids (euasterids), which comprise two large sister clades: euasterids I (lamiids) + euasterids II (campanulids). The Cornales and Ericales were not considered closely related to the Asteridae in previous classifications, and the families were typically placed instead in Rosidae and Dilleniidae, respectively. Euasterids are mostly united by flowers with epipetalous stamens that equal the number of corolla lobes and a gynoecium of two fused carpels.




Representatives of the asterid clade, including the flower of Triodanis perfoliata (Campanulaceae: Asterales: euasterids II; photo © Paul Corogin), the stem and flower of Conradina brevifolia (Lamiaceae: Lamiales; euasterids I; photo © Paul Corogin), and the flowers of Ceropegia sandersonii (Apocynaceae: Gentianales: euasterids I; photo © Matyas Buzgo)
Within the euasterids, the euasterid I (or lamiid, B. Bremer et al., 2002) and euasterid II (or campanulid, B. Bremer et al., 2002) clades are sisters and can be distinguished both morphologically and molecularly (Albach et al., 2001a; K. Bremer et al., 2001; B. Bremer et al., 2002). Most members of lamiids have opposite leaves, entire leaf margins, hypogynous flowers, "early sympetaly" with a ring-shaped primordium, fusion of stamen filaments to the corolla tube, and capsular fruits (K. Bremer et al., 2001). The lamiids comprise Garryales, Gentianales, Solanales, and Lamiales, plus Boraginaceae, Vahliaceae, and Oncothecaceae + Icacinaceae (APG II, 2003). Most campanuliids have alternate leaves, serrate-dentate leaf margins, epigynous flowers, "late sympetaly" with distinct petal primordia, free stamen filaments, and indehiscent fruits (K. Bremer et al., 2001). It is unclear which of the characters that distinguish euasterids I and II are truly synapomorphies for these clades and which are symplesiomorphies; both reversals and parallelisms have contributed to complex patterns of morphological evolution in the asterids (Albach et al., 2001b; K. Bremer et al., 2001). The campanuliids are composed of Dipsacales, Aquifoliales, Apiales, and Asterales, plus Bruniaceae + Columelliaceae, a small clade of Tribelaceae, Polyosmaceae, Escalloniaceae, and Eremosynaceae, and possibly Paracryphiaceae. The campanulids include families previously classified in Asteridae and Rosidae (Cronquist, 1981, 1988). The asterids are covered in more detail in Judd et al. (2002), Judd and Olmsead (2004), P. Soltis et al. (2004), P. Soltis and Soltis (2004) and D. Soltis et al. (2005).
More representatives of the asterid clade, including the flower of Berlandiera subacaulis (Asteraceae: Asterales: euasterids II; photo © Paul Corogin), the flowers of Buchnera subacaulis (Apocynaceae: Lamiales: euasterids I; photo © Paul Corogin), and the flowers of Cornus nuttallii (Cornaceae: Cornales; photo © Matyas Buzgo).
References
Angiosperm Phylogeny Group (APG). 1998. An ordinal classification for the families of flowering plants. Annals of the Missouri Botanical Garden 85: 531-553.
Angiosperm Phylogeny Group (APG II). 2003. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II. Botanical Journal of the Linnean Society 141: 399-436.
Barkman, T. J., G. Chenery, J. R. McNeal, J. Lyons-Weiler, and C. W. DePamphilis. 2000. Independent and combined analyses of sequences from all three genomic compartments converge on the root of flowering plant phylogeny. Proceedings of the National Academy of Sciences, USA 97: 13166-13171.
Barraclough, T. G., and V. Savolainen. 2001. Evolutionary rates and species diversity in flowering plants. Evolution 55:677-683.
Bell, C. D., D. E. Soltis, and P. S. Soltis. 2005. The age of the angiosperms: a molecular timescale without a clock. Evolution, 59: 1245-1257.
Bharathan, G., and E. A. Zimmer. 1995. Early branching events in monocotyledons-partial 18S ribosomal DNA sequence analysis. In P. J. Rudall, P. J., Cribb, D. F. Cutler, and C. J. Humphries [eds.], Monocotyledons: systematics and evolution, 81-107. Royal Botanic Gardens, Kew, London, UK.
Braun, A. 1864. Uebersicht der naturlichen Systems nach der Anordnung derselben. In P. Ascherson [ed.], Flora der Provinz Brandenburg, der Altmark und des Herzogthums Magdeburg, vol. 1, 22-67. Hirschwald, Berlin, Germany.
Bremer, K., A. Backlund, B. Sennblad, U. Swenson, K. Andreasen, M. Hjertson, J. Lundberg, M. Backlund, and B. Bremer. 2001. A phylogenetic analysis of 100+ genera and 50+ families of euasterids based on morphological and molecular data with notes on possible higher level morphological synapomorphies. Plant Systematics and Evolution 229: 137-169.
Burleigh, G. G., and S. Mathews. 2004. Phylogenetic signal in nucleotide data from seed plants: implications for resolving the seed plant tree of life. American. Journal of Botany. 91: 1599-1613.
Chase, M. W., and V. A. Albert. 1998. A perspective on the contribution of plastid rbcL DNA sequences to angiosperm phylogenetics. In D. E. Soltis, P. S. Soltis, and J. J. Doyle [eds.], Molecular systematics of plants, vol. 2: DNA sequencing, 488-507. Kluwer, Boston, Massachusetts, USA.
Chase, M. W., D. E. Soltis, R. G. Olmstead, D. Morgan, D. H. Les, B. D. Mishler, M. R. Duvall, R. A. Price, H. G. Hills, Y.-L. Qiu, K. A. Kron, J. H. Rettig, E. Conti, J. D. Palmer, J. R. Manhart, K. J. Sytsma, H. J. Michaels, W. J. Kress, K. G. Karol, W. D. Clark, M. Hedren, B. S. Gaut, R. K. Jansen, K.-J. Kim, C. F. Wimpee, J. F. Smith, G. R. Furnier, S. H. Strauss, Q.-Y. Xiang, G. M. Plunkett, P. S. Soltis, S. M. Swensen, S. E. Williams, P. A. Gadek, C. J. Quinn, L. E. Eguiarte, E. Golenberg, G. H. Learn, Jr., S. W. Graham, S. C. H. Barrett, S. Dayanandan, and V. A. Albert. 1993. Phylogenetics of seed plants: an analysis of nucleotide sequences from the plastid gene rbcL. Annals of the Missouri Botanical Garden 80: 528-580.
Chaw, S. M., C. C. Chang, H. L. Chen, and W. H. Li. 2004. Dating the monocot-dicot divergence and the origin of core eudicots using whole chloroplast genomes. Journal of Molecular Evolution 58: 424-441.
Clement, J. S., and T. J. Mabry. 1996. Pigment evolution in the Caryophyllales: a Systematic Overview. Botanica Acta 109: 360-367.
Crepet, W., and K. Nixon. 1998. Fossil Clusiaceae from the Late Cretaceous (Turonian) of New Jersey and implications regarding the history of bee pollination. American Journal of Botany 85: 1122-1133.
Cronquist, A. 1981. An integrated system of classification of flowering plants. Columbia University Press, New York, USA.
Cronquist, A. 1988. The evolution and classification of flowering plants, 2nd ed. New York Botanical Garden, Bronx, New York, USA.
Dahlgren, R. 1980. A revised system of classification of the angiosperms. Botanical Journal of the Linnean Society 80: 91-124.
Davies, T. J., T. G. Barraclough, M. W. Chase, P. S. Soltis, D. E. Soltis, and V. Savolainen. 2004. Darwin's abominable mystery: insights from a supertree of the angiosperms. Proceedings of the National Academy of Sciences, USA 101: 1904-1909.
Davis, C. C., and M. W. Chase. 2004. Elatinaceae are sister to Malpighiaceae; Peridiscaceae belong to Saxifragales. American Journal of Botany 91: 262-273.
DeCraene, L. P. R. 2004. Floral development of Berberidopsis corallina: a crucial link in the evolution of flowers in the core eudicots. Annals of Botany 94: 741-751.
De Jussieu, A. L. 1789. Genera plantarum secundum ordines naturals disposita. Heissant and Barrois, Paris, France.
Donoghue, M. J., and J. A. Doyle. 1989. Phylogenetic studies of seed plants and angiosperms based on morphological characters. In K. Bremer and H. Jörnvall [eds.], The hierarchy of life: molecules and morphology in phylogenetic studies, 181-193. Elsevier Science Publishers, Amsterdam, The Netherlands.
Douglas, A., and S. C. Tucker. 1996. Comparative floral ontogenies among Persoonioideae including Bellendena (Proteaceae). American Journal of Botany 83: 1528-1555.
Doyle, J. A. 1998. Phylogeny of vascular plants. Annual Review of Ecology and Systematics 29:567-599.
Doyle, J. A., and M. J. Donoghue. 1986. Seed plant phylogeny and the origin of the angiosperms: an experimental cladistic approach. Botanical Review 52: 321-431.
Doyle, J. A., and M. J. Donoghue. 1992. Fossils and seed plant phylogeny reanalyzed. Brittonia 44: 89-106.
Doyle, J. A., and M. J. Donoghue. 1993. Phylogenies and angiosperm diversification. Paleobiology 19:141-167.
Doyle, J. A., M. J. Donoghue, and E. A. Zimmer. 1994. Integration of morphological and rRNA data on the origin of angiosperms. Annals of the Missouri Botanical Garden 81:419-450.
Drinnan, A. N., P. R. Crane, and S. B. Hoot. 1994. Patterns of floral evolution in the early diversification of non-magnoliid dicotyledons (eudicots). Plant Systematics and Evolution 8 (Supplement): 93-122.
Eichler, A. 1875-1878. Blüthendiagramme I/II. Engelmann, Leipzig, Germany.
Gottsberger, G. 1977. Some aspects of beetle pollination in the evolution of flowering plants. Plant Systematics and Evolution 1: 211-226.
Heywood, V. 1993. Flowering plants of the world. B.T. Batsford Ltd., London, UK.
Hickey, L. J., and A. D. Wolfe. 1975. The bases of angiosperm phylogeny: vegetative morphology. Annals of the Missouri Botanical Garden 62: 538-589.
Hilu, K. W., T. Borsch, K. Muller, D. E. Soltis, P. S. Soltis, V. Savolainen, M. Chase, M. Powell, L. Alice, R. Evans, H. Sauquet, C. Neinhuis, T. Slotta, J. Rohwer, and L. Chatrou. 2003. Inference of angiosperm phylogeny based on matK sequence information. American Journal of Botany 90: 1758-1776.
Hoot, S. B., S. Magallón, and P. R. Crane. 1999. Phylogeny of basal eudicots based on three molecular data sets: atpB, rbcL, and 18S nuclear ribosomal DNA sequences. Annals of the Missouri Botanical Garden 86: 1-32.
Hufford, L. 1992. Rosidae and their relationships to other nonmagnoliid dicotyledons: A phylogenetic analysis using morphological and chemical data. Annals of the Missouri Botanical Garden 79: 218-248.
Judd, W. S., C. S. Campbell, E. A. Kellogg, P. F. Stevens, and M. J. Donoghue. 2002. Plant systematics: a phylogenetic approach. Sinauer Associates, Inc., Sunderland, Massachusetts, USA.
Judd, W. S., and R. G. Olmstead. 2004. A survey of tricolpate (eudicot) phylogenetic relationships. American Journal of Botany 91: 1627-1644.
Kallersjo, M., J. S. Farris, M. W. Chase, B. Bremer, M. F. Fay, C. J. Humphries, G. Pedersen, O. Seberg, and K. Bremer. 1998. Simultaneous parsimony jackknife analysis of 2538 rbcL DNA sequences reveals support for major clades of green plants, land plants, seed plants and flowering plants. Plant Systematics and Evolution 213:259-287.
Kim, S., D. E. Soltis, P. S. Soltis, M. J. Zanis, and Y. Suh. 2004. Phylogenetic relationships among early-diverging eudicots based on four genes: were the eudicots ancestrally woody? Molecular Phylogenetics and Evolution 31: 16-30.
Kramer, E. M., R. L. Dorit, and V. F. Irish. 1998. Molecular evolution of genes controlling petal and stamen development: Duplication and divergence within the APETALA3 and PISTILLATA MADS-box gene lineages. Genetics 149: 765-783.
Kuzoff, R. K., L. Hufford, and D. E. Soltis. 2001. Structural homology and developmental transformations associated with ovary diversification in Lithophragma (Saxifragaceae). American Journal of Botany 88: 196-205.
Litt, A., and V. Irish. 2003. Duplication and diversification in the APETALA1/FRUITFULL floral homeotic gene lineage: implications for the evolution of floral development. Genetics 165: 821-833.
Mabberley, D. J. 1993. The plant book: a portable dictionary of the vascular plants. Cambridge University Press, Cambridge, UK.
Magallón, S., P. R. Crane, and P. S. Herendeen. 1999. Phylogenetic pattern, diversity, and diversification of eudicots. Annals of the Missouri Botanical Garden 86: 297-372.
Magallón, S., and M. J. Sanderson. 2001. Absolute diversification rates in angiosperm clades. Evolution 55: 1762-1780.
Magallón, S., and M. J. Sanderson. 2005. Angiosperm divergence times: the effect of genes, codon positions, and time constraints. Evolution 59: 1653-1670.
Martin, W., D. Lydiate, H. Brinkmann, G. Forkmann, H. Saedler, and R. Cerff. 1993. Molecular phylogenies in angiosperm evolution. Molecular Biology and Evolution 10:140-162.
Nandi, O. I., M. W. Chase, and P. K. Endress. 1998. A combined cladistic analysis of angiosperms using rbcL and nonmolecular data sets. Annals of the Missouri Botanical Garden 85: 137-212.
Nickerson, J., and G. Drouin. 2004. The sequence of the largest subunit of RNA polymerase II is a useful marker for inferring seed plant phylogeny. Molecular Phylogenetics and Evolution 31: 403-415.
Nickrent, D. L., R. J. Duff, A. Colwell, A. D. Wolfe, N. D. Young, K. E. Steiner, and C. W. DePamphilis. 1998. Molecular phylogenetic and evolutionary studies of parasitic plants.In D. E. Soltis, P. S. Soltis, and J. J. Doyle [eds.], Molecular systematics of plants, vol. 2: DNA sequencing, 488-507. Kluwer, Boston, Massachusetts, USA.
Nickrent, D. L., and V. Malécot. 2001. A molecular phylogeny of Santalales. Pages 69-74 in A. Fer, P. Thalouarn, D. M. Joel, L. J. Musselman, C. Parker, and J. A. C. Verkleij, eds. Proceedings of the 7th. International Parasitic Weed Symposium. Faculté des Sciences, Université de Nantes, Nantes, France.
Nickrent, D. L., and D. E. Soltis. 1995. A comparison of angiosperm phylogenies from nuclear 18S rDNA and rbcL sequences. Annals of the Missouri Botanical Garden 82:208-234.
Parkinson, C. L., K. L. Adams, and J. D. Palmer. 1999. Multigene analyses identify the three earliest lineages of extant flowering plants. Current Biology 9: 1485-1488.
Oxelman, B., N. Yoshikawa, B. L. McConaughy, J. Luo, A. L. Denton, and B. D. I. Hall. 2004. RPB2 gene phylogeny in flowering plants, with particular emphasis on asterids. Molecular Phylogenetics and Evolution 32: 462-479.
Pyankov, V. I., E. G. Artyusheva, G. E. Edwards, C. C. J. Black, and P. S. Soltis. 2001. Phylogenetic analysis of tribe Salsoleae (Chenopodiaceae) based on ribosomal ITS sequences: implications for the evolution of photosynthesis types. American Journal of Botany 88: 1189-1198.
Reichenbach, H. G. L. 1827-1829. Dr. Joh. Christ, Moessler's Handbuch der Gewaechskunde, [ed.] vols. 2, 3. Hammerich, Altona, Germany.
Rodman, J. E., P. S. Soltis, D. E. Soltis, K. J. Sytsma, and K. G. Karol. 1998. Parallel evolution of glucosinolate biosynthesis inferred from congruent nuclear and plastid gene phylogenies. American Journal of Botany 85: 997-1006.
Sanderson, M. J., and M. J. Donoghue. 1994. Shifts in diversification rate with the origin of angiosperms. Science 264:1590-1593.
Sanderson, M., J., and J. A. Doyle. 2001. Sources of error and confidence intervals in estimating the age of angiosperms from rbcL and 18S rDNA data. American Journal of Botany 88: 1499-1516.
Savolainen, V., M. W. Chase, C. M. Morton, D. E. Soltis, C. Bayer, M. F. Fay, A. De Bruijn, S. Sullivan, and Y.-L. Qiu. 2000a. Phylogenetics of flowering plants based upon a combined analysis of plastid atpB and rbcL gene sequences. Systematic Biology 49: 306-362.
Savolainen, V., M. F. Fay, D. C. Albach, A. Backlund, M. Van Der Bank, K. M. Cameron, S. A. Johnson, M. D. Lledó, J.-C. Pintaud, M. Powell, M. C. Sheahan, D. E. Soltis, P. S. Soltis, P. Weston, W. M. Whitton, K. J. Wurdack, and M. W. Chase. 2000b. Phylogeny of the eudicots: a nearly complete familial analysis based on rbcL gene sequences. Kew Bulletin 55: 257-309.
Soltis, D. E., P. S. Soltis, D. R. Morgan, S. M. Swensen, B. C. Mullin, J. M. Dowd, and P. G. Martin. 1995. Chloroplast gene sequence data suggest a single origin of the predisposition for symbiotic nitrogen fixation in angiosperms. Proceedings of the National Academy of Sciences, USA 92: 2647-2651.
Soltis, D. E., P. S. Soltis, D. L. Nickrent, L. A. Johnson, W. J. Hahn, S. B. Hoot, J. A. Sweere, R. K. Kuzoff, K. A. Kron, M. W. Chase, S. M. Swensen, E. A. Zimmer, S.-M. Chaw, L. J. Gillespie, W. J. Kress, and K. J. Sytsma. 1997. Angiosperm phylogeny inferred from 18S ribosomal DNA sequences. Annals of the Missouri Botanical Garden 84: 1-49.
Soltis, D. E., P. S. Soltis, M. E. Mort, M. W. Chase, V. Savolainen, S. B. Hoot, and C. M. Morton. 1998. Inferring complex phylogenies using parsimony: an empirical approach using three large DNA data sets for angiosperms. Systematic Biology 47: 32-42.
Soltis, D. E., P. S. Soltis, M. W. Chase, M. E. Mort, D. C. Albach, M. Zanis, V. Savolainen, W. J. Hahn, S. B. Hoot, M. F. Fay, M. Axtell, S. M. Swensen, L. M. Prince, W. J. Kress, K. C. Nixon, and J. S. Farris. 2000. Angiosperm phylogeny inferred from 18S rDNA, rbcL, and atpB sequences. Botanical Journal of the Linnean Society 133: 381-461.
Soltis, D. E., A. E. Senters, M. Zanis, S. Kim, J. D. Thompson, P. S. Soltis, L. P. Ronse De Craene, P. K. Endress, and J. S. Farris. 2003. Gunnerales are sister to other core eudicots: implications for the evolution of pentamery. American Journal of Botany 90: 461-470.
Soltis, D. E., P. S. Soltis, M. W. Chase, and P. K. Endress. 2005. Angiosperm phylogeny and evolution. Sinauer Associates, Inc., Sunderland, Massachusetts, USA.
Soltis, D. E., V. A. Albert, V. Savolainen, K. W. Hilu, Y-L. Qiu, M. W. Chase, J., S. Farris, S. Stefanovic, D. W. Rice, J. D. Palmer, and P. S. Soltis. 2004. Genome-scale data, angiosperm relationships, and "ending incongruence": a cautionary tale in phylogenetics. Trends in Plant Science, 9: 477-483.
Soltis, P. S., D. E. Soltis, and M. W. Chase. 1999. Angiosperm phylogeny inferred from multiple genes as a tool for comparative biology. Nature 402: 402-404.
Soltis, P. S., D. E. Soltis, M. W. Chase, P. K. Endress, and P. R. Crane. 2004. The diversification of flowering plants. In J. Cracraft and M. J. Donoghue [eds.], The tree of life. Oxford University Press, Oxford, UK.
Stebbins, G. L. 1974. Flowering plants: evolution above the species level. Harvard University Press, Cambridge, Massachusetts, USA.
Swensen, S. M. 1996. The evolution of actinorhizal symbioses: evidence for multiple origins of the symbiotic association. American Journal of Botany 83: 1503-1512.
Sytsma, K. J., and D. A. Baum. 1996. Molecular phylogenies and the diversification of angiosperms. In D. W. Taylor and L. J. Hickey [eds.], Flowering plant origin, evolution, and phylogeny, 314-340. Chapman and Hall, New York, NY, USA.
Takhtajan, A. 1980. Outline of the classification of flowering plants (Magnoliophyta). Botanical Review 46: 225-359.
Takhtajan, A. 1987. System of Magnoliophyta. Academy of Sciences, Leningrad, USSR.
Takhtajan, A. 1991. Evolutionary trends in flowering plants. Columbia University Press, New York, NY, USA.
Takhtajan, A. 1997. Diversity and classification of flowering plants. Columbia University Press, New York, NY, USA.
Taylor, D., and L. Hickey. 1992. Phylogenetic evidence for the herbaceous origin of angiosperms. Plant Systematics and Evolution 180: 137-156.
Thorne, R. F. 1974. A phylogenetic classification of the Annoniflorae. Aliso 8: 147-209.
Thorne, R. F. 1992. Classification and geography of the flowering plants. Botanical Review 58: 225-348.
van Tieghem, P. 1897. Sur les Buxaées. Annales des Sciences Naturelles Botanique. Série 8, 5: 289-338.
Warming, E. 1879. Haandbog I den systematiske botanik. P. G. Philipsens Forlag, Copenhagen, Denmark.
Wikström, N., V. Savolainen, and M. W. Chase. 2001. Evolution of the angiosperms: calibrating the family tree. Proceedings of the Royal Society of London, B 268: 2211-2220.
Zimmer, E. A., R. K. Hamby, M. L. Arnold, D. A. LeBlanc and E. C. Theriot. 1989. Ribosomal RNA phylogenies and flowering plant evolution. In B. Fernholm, K. Bremer and H. Jornvall [eds.], The hierarchy of life, Pp. 205-214. Elsevier, Amsterdam, the Netherlands.
Information on the Internet
- Angiosperm Phylogeny Website. P. F. Stevens, Missouri Botanical Garden.
- Deep Time Project: A Comprehensive Phylogenetic Tree of Living and Fossil Angiosperms. Florida Museum of Natural History.
- The Families of Flowering Plants. L. Watson and M. J. Dallwitz.
- Images and Descriptions of Flowering Plant Families (as treated by Arthur Cronquist). University of Hawaii Botany Department.
- Flowering Plant Gateway. Hugh Wilson, Texas A&M Bioinformatics Working Group.
- Classification of Flowering Plants. Kåre Bremer, Birgitta Bremer & Mats Thulin, Uppsala University.
- Thonner's analytical key to the families of flowering plants.
Title Illustrations

| Scientific Name | Ferocactus cylindraceus |
|---|---|
| Location | Palm Springs, Chino Canyon (Riverside County, California, USA) |
| Comments | Caryophyllales, Cactaceae |
| Creator | Photograph by Marguerite Gregory |
| Specimen Condition | Live Specimen |
| Source | Ferocactus cylindraceus; Biznaga-barril Cilíndrica |
| Source Collection | CalPhotos |
| Copyright |
© 1999 California Academy of Sciences

|
| Scientific Name | Catananche caerulea |
|---|---|
| Location | cultivated at Schweiz, Bot. Garten Basel |
| Creator | H. Schneider |
| Specimen Condition | Live Specimen |
| Source | Catananche caerulea (Asteraceae) |
| Source Collection | Botanical Image Database |
| Copyright |
© 2001 University of Basel, Basel, Switzerland

|
| Scientific Name | Populus tremuloides |
|---|---|
| Location | Silverton (Colorado, US) |
| Comments | Rosids, Salicaceae |
| Creator | Photograph by Charles Webber |
| Specimen Condition | Live Specimen |
| Source | Populus tremuloides; Quaking Aspen |
| Source Collection | CalPhotos |
| Copyright |
© 2000 California Academy of Sciences

|
| Scientific Name | Ampelopsis brevipedunculata |
|---|---|
| Location | cultivated, Asheville, North Carolina, USA |
| Comments | This fast-growing alien from Northeast Asia, is in the Grape family (Vitaceae). |
| Specimen Condition | Live Specimen |
| Source | porcelainberries |
| Source Collection | Flickr |
| Image Use |
 This media file is licensed under the Creative Commons Attribution-NonCommercial-ShareAlike License - Version 2.0. This media file is licensed under the Creative Commons Attribution-NonCommercial-ShareAlike License - Version 2.0.
|
| Copyright | © 2008 zen Sutherland |
About This Page
Thanks to Walter Judd, Paul Corogin, Sangtae Kim, Matyas Buzgo, and Scott Zona for use of their wonderful images.
Doug Soltis

Department of Botany and the Genetics Institute, Gainesville, Florida, USA

Florida Museum of Natural History and the Genetics Institute, Gainesville, Florida, USA

Department of Botany, University of Wyoming, Laramie, Wyoming, USA
Correspondence regarding this page should be directed to Christine Edwards at
Page copyright © 2005 , , and
All Rights Reserved.
Citing this page:
Soltis, Doug, Pam Soltis, and Christine Edwards. 2005. Core Eudicots. Core Tricolpates. Version 01 January 2005. http://tolweb.org/Core_Eudicots/20714/2005.01.01 in The Tree of Life Web Project, http://tolweb.org/

















 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site