Characiformes
Tetras, piranhas, hatchetfishes, headstanders, pencilfishes, and their relatives
Guillermo Ortí and Richard P. Vari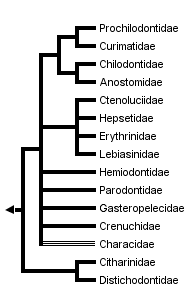


This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxThe family Characidae, whose members live in Africa and from Texas in North America through Central and South America, is probably non-monophyletic.
Introduction
The order Characiformes includes a vast array of fishes that live in rivers and lakes of Africa and the New World (from Texas in North America through Central and South America). They are divided into 14 or 16 families (Géry, 1977, and Greenwood et al., 1966, respectively), four of which are African (over 200 species), and the rest live mostly in the Neotropics (more than 1,200 species). They include well-known forms like piranhas, tetras (e.g. neon tetras, silver dollars), hatchetfishes, and pencilfishes, popular in the aquarium trade. Other characiforms also have commercial importance as a food resource for human communities living along the banks of broad tropical rivers. These include the African citharinids and the Neotropical prochilodontids, which are very abundant, large-bodied species, typically forming massive schools that migrate up and down the main river channels. Other characid groups (family Characidae) include important sport-fishing species, like the african tiger fish (genus Hydrocynus, subfamily Alestiinae), and the Neotropical dorados (genus Salminus, subfamily Bryconinae). These are voracious predators that may reach a size of 100-130 cm in length and a weight of up to 50 Kg. In contrast, the smallest characiform species, known as miniature forms (Weitzman and Vari, 1988), have adults that do not exceed 26 mm in length (some tetras, glandulocaudines, and lebiasinids). The only comprehensive account of the order Characiformes as a whole published to date is that of Géry (1977).
The variety of ecological specializations found among characiforms is remarkable. The range of feeding modes varies from detritivory (mud-eating prochilodontids and curimatids), herbivory (plant-eating citharinids and anostomids), and planktivory (plankton-filtering, e.g. Anodus and Clupeocharax), to predation (Hepsetus, Hoplias, Hydrocynus and Salminus), fin-eating and scale-eating (some distichodontids and characids), and to the notorious and voracious group-predation of some piranhas.
Peculiar morphological and physiological adaptations permit the survival of some groups in extreme conditions of low oxygen concentration, typical of flood-plain environments (e.g., erythrinids are capable of air-breathing, and some pacus, ctenolucids, and leporins have swollen lips that allow them to exchange gases in the uppermost film of water). Figure 1 shows a picture of Ctenolucius doing this. Some characiform groups exhibit nesting behaviors and parental care (erythrinids, Hepsetus, piranhas, and lebiasinids). The Characiformes are all primary freshwater fishes, unable to survive in saline environments.


Figure 1. Ctenolucius hujeta (dorsal view) from the Maracaibo basin (Venezuela). Membranes of the lips are clearly extended posteriorly, preceded by a thickened margin of skin. In this way, fish adapted to poor oxygen concentration in the water, a typical condition in flood plain enironments, may "skim" the upper layer of the water for oxygenation. The fish was being held in a bucket of water. Photo copyright © John Lundberg.
Origin of the major characiform lineages has been dated to more than 100 million years ago, when Africa and South America still formed part of a single continental mass called Gondwana (Lundberg 1993; Ortí and Meyer 1997). Given the paucity of direct fossil evidence, this inference has been based on indirect evidence such as the phylogenetic and geographic distribution of extant characiform families and the genetic distances separating them. The alternative view, suggesting that most lineages had originated from a few ancestral forms independently in each continent after the separation of Africa and South America (some 80-100 million years ago), is not consistent with the available evidence. Under this view, all African and all Neotropical families should form reciprocally monophyletic groups. But as shown above in the characiform tree, the African families (Citharinidae, Distichodontidae, Hepsetidae, and part of Characidae) are dispersed among the Neotropical families rather than forming a single monophyletic unit. Also, genetic distances among African characiforms and their closest Neotropical relatives are not significantly larger than genetic distances among families within each continent. The primary diversification of charciform fishes is thus placed in the Gondwanan continent, more than 100 million years ago.
Characteristics
Characiforms usually have an adipose fin (Figure 2) and almost always, well developed scales (the only naked characin is Gymnocharacinus bergi from Argentina, also the most southerly distributed species). Teeth are also usually present since most are carnivores or omnivores. Pelvic fins are present (with 5-12 rays) and the anal fin is short to moderately long (fewer than 45 rays). The lateral line is often decurved and sometimes incomplete. Barbels are always absent. Characiformes share with other otophysan fishes a strucuture called the Weberian apparatus, which is a distinctive modification of the anterior-most four or five vertebrae. It contains a series of movable bony parts (ossicles) and ligaments that connect the swim bladder to the inner ear, presumably for effcient sound transmission.

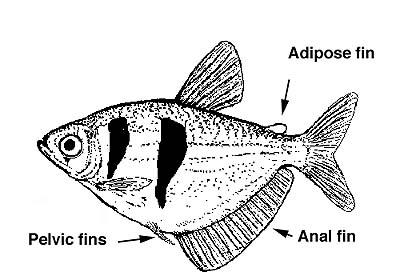
Figure 2. Gymnocorymbus ternetzi, the black tetra, showing the disposition of the fins.
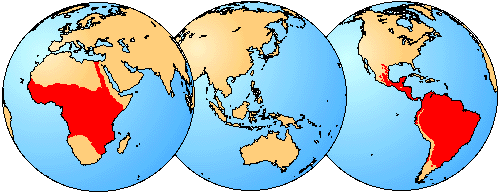
Geographic Distribution
Characiformes are exclusively freshwater fishes that live in streams, rivers, and lakes (marked red). Distribution in Africa taken from Daget et al. (1984).

Discussion of Phylogenetic Relationships
The division of the order Characiformes into families is a controversial matter, in part because not all of the proposed groups have been shown to be monophyletic. Many well-characterized lineages are currently lumped into the family Characidae, which may not form a natural group (Weitzman and Fink, 1983, but see our discussion of Lucena, 1993). For the present discussion, we will use a family-group classification that follows Greenwood et al. (1966), with a few exceptions: the family Cynodontidae is included in the family Characidae, as suggested by Howes (1976); the ichthyborids are included within the family Distichodontidae, following Vari (1979); and the characid subfamilies Characidiinae and Crenuchinae are grouped into the new family Crenuchidae (following Buckup, 1991). Provisionally, we recognize 15 characiform families.
The establishment of higher-order phylogenetic relationships among characiform families has been difficult and controversial. Following a conservative approach, we present a mostly unresolved phylogeny rather than any of the alternative hypotheses published to date. The groupings shown are supported by or consistent with both morpological and molecular evidence.
The only comprehensive phylogenetic study to date to include all putative characiform families (and several lineages from the family Characidae) was based on mitochondrial DNA sequence data (Ortí and Meyer, 1997). The molecular data, however, fell short of providing a robust hypothesis of relationsips among characiform families, except for a few clades also suggested by previous morphological evidence (some of the DNA sequence-based phylogenetic trees published are shown here). Several pylogenetic studies based on morphology have targeted lower-level relationships within families (e.g., Vari, 1988; Weitzman et al., 1988; Buckup, 1993; Langeani, 1996) or a subset of closely-related lineages (Vari, 1979, 1983, 1995). More inclusive studies were presented by Buckup (1991) and Uj (1990). A summary of the most relevant hypotheses based on morphological data sets is shown in Figure 3.

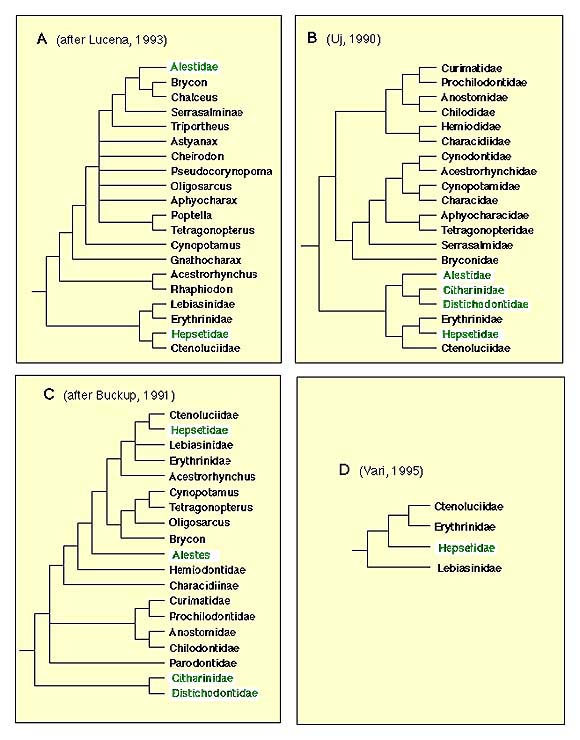
Figure 3. Phylogenetic trees based on morphological characters. None of the them include all extant families of Characiformes. African taxa are shown in green letters.
The African families Citharinidae and Distichodontidae were shown to be monophyletic and to form a well-supported monophyletic unit by Vari (1979). Their basal position in the Characiformes, originally suggested by Fink and Fink (1981), was corrobarated by Buckup (1991) and by molecular evidence.
Monophyly of the families Curimatidae, Prochilodontidae, Anostomidae, and Chilodontidae was proposed by Vari (1983). He also hypothesised a monophyletic unit composed by these four families, later corroborated by Buckup (1991; see figure 3C). The molecular evidence strongly supports a sister-group relationship between prochilodontids and curimatids, and is also consistent with a sister-group relationship between Anostomids and Chilododontids. Relationships between these latter two units remains poorly resolved by the mtDNA data.
The assemblage formed by the families Hepsetidae, Erythrinidae, Ctenoluciidae, and Lebiasinidae was suggested by Buckup (1991), and later corroborated by Vari (1995). Although a critical appraisal of the question of the monophyly of the families Lebiasinidae, Erythinidae, and Hepsetidae is lacking, Vari (1995) listed some putative synapomorphic characters for each of these units in his analysis of the Ctenoluciidae. Monophyly of the Ctenoluciidae was well supported by 22 synapomorphies (Vari 1995). Hypotheses of relationships among these four familes differ between studies (compare Figure 3C and D). The molecular data supports a sister-group relationship between Hepsetidae and Erythrinidae.
Buckup (1991, 1993) proposed a monophyletic family Crenuchidae, which includes the Characidiinae and Crenuchinae. These units were previosuly part of the family Characidae. Langeani (1996) reported 17 synapomorphies that support the hypothesis of monophyly of the family Hemiodontidae. No detailed phylogenetic analysis of the family Parodontidae has been published, but information provided by Roberts (1974) and Starnes and Schindler (1993) support the monophyly of Parodontidae (see Starnes and Schindler, 1993:755). Finally, Weitzman (1954) provided a detailed description of the osteology of the family Gasteropelecidae. Although that study long predated the advent of cladistic methodology, a number of features of this distinctive family noted by Weitzman are undoubtedly synapomorphies for this group.
Other Names for Characiformes
- Tetras, piranhas, hatchetfishes, headstanders, pencilfishes, and their relatives
References
Buckup, P. A. 1991. The Characidiinae: A phylogenetic study of the South American darters and their relationships with other characiform fishes. Ph.D., The University of Michigan, Ann Arbor, USA.
Buckup, P. A. 1993. Phylogenetic interrelationships and reductive evolution in Neotropical characidiin fises (Characiformes, Ostariopysi). Cladistics 9:305-341.
Daget, J.,J.-P. Gosse, and D.F.E. Thys van den Audenaerde. 1984. Check-list of the freshwater fishes of Africa (Cloffa). Volume I, pp. 138-216. ORSTOM and MRAC, Paris and Tervuren.
Géry, J. 1977. Characoids of the World. ed. Tropical Fish Hobbyist Publications, Neptune City, New Jersey.
Greenwood, P. H., D. E. Rosen, S. H. Weitzman, and G. S. Myers. 1966. Phyletic studies of teleostean fishes, with a provisional classification of living forms. Bulletin of the American Museum of Natural History 131:339-354.
Howes, G. J. 1976. The cranial musculature and taxonomy of the tribes Cynodontini and Characini. Bull. British, Museum (Nat. Hist.), Zoology 29:203-248.
Langeani, F. 1996. Estudio filogenético e revisão da família Hemiodontidae Boulenger, 1904 (sensu Roberts, 1974) (Ostariophysi, Characiformes), Unpublished Ph.D. thesis, Universidade de São Paulo, Brasil.
Lucena, C. A. S. d. 1993. Estudo filogenético da família Characidae com uma discussão dos grupos naturais propostos (Teleostei, Ostariophysi, Characiformes). Unpublished Ph.D. thesis, Universidade de São Paulo, Brasil.
Lundberg, J. G. 1993. African-South American freshwater fish clades and continental drift: problems with a paradigm, p. 156-199. In: Biological relationships between Africa and South America. P. Goldblatt (eds.). Yale University Press, New Haven.
Ortí, G. 1997. The Radiation of Characiform Fishes: Evidence from Mitochondrial and Nuclear DNA Sequences. Pp. 215-239 In: “Molecular Systematics of Fishes”, T.D. Kocher and C. Stepien Eds., Academic Press, San Diego, California.
Ortí, G.and A. Meyer. 1997. The radiation of characiform fishes and the limits of resolution of mitochondrial ribosomal DNA sequences. Syst. Biol. 46:75-100.
Ortí, G. and A. Meyer. 1996. Molecular evolution of ependymin and the phylogenetic resolution of early divergences among teleost fishes. Molecular Biology and Evolution 13 (4): 556-573.
Ortí, G., P. Petry, J. A. Porto, M. Jegú, and A. Meyer. 1996. Patterns of nucleotide change in mitochondrial ribosomal RNA genes and the phylogeny of piranhas. Journal of Molecular Evolution 42 (2): 169-182.
Roberts, T.R. 1974. Osteology and classification of the Neotropical characoid fishes of the families Hemiodontidae (including Anostomidae) and Parodontidae. Bulletin of the Museum of Comparative Zoology, 146 (9): 411-472.
Starnes, W.C. and I. Schindler. 1993. Comments on the genus Apareiodon Eigenmann (Characiformes: Parodontidae) with the description of a new species from the Gran Sabana region of eastern Venezuela. Copeia 1993 (3): 754-762.
Uj, A. 1990. Etude comparative de l'osteologie cranienne des poissons de la famille des Characidae et son importance phylogenetique. Unpublished Ph.D. thesis, Université de Geneve.
Vari, R. P. 1979. Anatomy, relationships, and classification of the families Citharinidae and Distichodontidae (Pisces, Characoidea). Bulletin of the British Museum (Natural History), Zoology 36:261-344.
Vari, R. P. 1983. Phylogenetic relationships of the families Curimatidae, Prochilodontidae, Anostomidae, and Chilodontidae. Smithsonian Contributions to Zoology 378:1-60.
Vari, R. P. 1988. The Curimatidae, a Lowland Neotropical fish family (Pisces: Characiformes); Distribution, Endemism, and Phylogenetic Biogeography. Pp. 313-348 in Neotropical Distribution Patterns: Proceedings of a 1987 Workshop (W. R. Heyer and P. E. Vanzolini, ed.). Academia Brasileira de Ciencias, Rio de Janeiro.
Vari, R. P. 1995. The Neotropical fish familiy Ctenoluciidae (Teleostei: Ostariophysi: Characiformes): supra and intrafamilial phylogenetic relationships, with a revisionary study. Smithsonian Contributions to Zoology 564:1-97.
Weitzman, S. H. 1954. The osteology and relationships of the South American characid fishes of the subfamily Gasteropelecidae. Stanford Ichthyological Bulletin, 4 (4): 213-263.
Weitzman, S. H.and W. L. Fink. 1983. Relationships of the neon tetras, a group of South American freshwater fishes (Teleostei, Characidae), with comments on the phylogeny of New World Characiformes. Bulletin of the Museum of Comparative Zoology 150:339-395.
Weitzman, S. H., N. Menezes, and M. J. Weitzman. 1988. Phylogenetic biogeography of the Glandulocaudini (Teleostei: Characiformes, Characidae) with comments on the distributions of other freshwater fishes in Eastern and Southeastern Brazil. Pp. 379-427 in Neotropical Distribution Patterns: Proceedings of a 1987 Workshop (W. R. Heyer and P. E. Vanzolini, ed.). Academia Brasileira de Ciencias, Rio de Janeiro.
Weitzman, S. H.and R. P. Vari. 1988. Miniaturization in South American freshwater fishes: an overview and discussion. Proc. Biol. Soc. Wash. 101:444-465.
Title Illustrations

| Scientific Name | Pygocentrus nattereri |
|---|---|
| Comments | Red-bellied piranha |
| Acknowledgements | Photo taken at the Tennessee Aquarium (Chattanooga, TN, USA), reproduced with permission. |
| Copyright | © 1997 E. Lasky and C. Smith |
About This Page

The George Washington University, Washington DC, USA

National Museum of Natural History, Smithsonian Institution, Washington, D. C., USA
Correspondence regarding this page should be directed to Guillermo Ortí at
Page copyright © 1997 and
All Rights Reserved.
- First online 27 January 1997
Citing this page:
Ortí, Guillermo and Richard P. Vari. 1997. Characiformes. Tetras, piranhas, hatchetfishes, headstanders, pencilfishes, and their relatives. Version 27 January 1997 (under construction). http://tolweb.org/Characiformes/15062/1997.01.27 in The Tree of Life Web Project, http://tolweb.org/









 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site