Himantolophidae
Himantolophus
Footballfishes
Theodore W. Pietsch

This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
Himantolophids are among the most easily recognized taxa of the suborder: with their globose spiny bodies, short, stout illicia, elaborately decorated escae, and protruding chins covered with numerous wart-like swellings, they can hardly be confused with anything else. One genus, five species groups, and 18 species are currently recognized. Specimens often attain lengths over 40 cm standard length.
Characteristics
Diagnosis
Metamorphosed females and males of the family Himantolophidae are distinguished from those of all other ceratioid families by lacking parietal bones throughout life (lost during metamorphosis in females of the gigantactinid genus Rhynchactis; see Bertelsen et al., 1981:10, fig. 13) and in having a broad toothless vomer, and triradiate pelvic bones (triradiate pelvics are also found in some specimens of the oneirodid genus Chaenophryne; see Pietsch, 1975:79, fig. 2).
Metamorphosed females and males are further unique in having the following combination of character states: supraethmoid, pterosphenoid, metapterygoid, and mesoperygoid present; hyomandibular with a double head; hypohyals 2; branchiostegal rays 6 (2 + 4); opercle bifurcate; subopercle with slender tapering dorsal prolongation, ventral end rounded, without anterior spine or projection; quadrate, articular, angular, and preopercular spines absent; postmaxillary process of premaxilla absent; pharyngobranchial I reduced in females, rudimentary in males; pharyngobranchials II and III present, toothed in females, toothless in males; pharyngobranchial IV absent; epibranchial I present; hypobranchial III absent; a single ossified basibranchial; epibranchial and ceratobranchial teeth present (only minute remnants of ceratobranchial teeth present in males; see below); epurals absent; hypural plate entire, without posterior notch; posteroventral process of coracoid absent; pectoral radials 3; dorsal-fin rays 5-6; anal-fin rays 4; pectoral-fin rays 14-18; pelvics absent; caudal-fin rays 9 (1 simple + 6 bifurcated + 2 simple), 9th (ventralmost) caudal ray well developed, more than half length of 8th; skin covered with dermal spines or spinules (spines hypertrophied in females; see below).
Metamorphosed females are further differentiated by having low rounded wart-like papillae covering the snout and chin; and large conical dermal spines, with extremely broad rounded bases widely spaced and scattered over the head and body. They differ further in having the following combination of character states: frontal bones widely separated, but without ventromedial extensions; sphenotic spines present; short; jaws subequal, lower jaw extending anteriorly beyond upper; lower jaw with well-developed symphysial spine; anterior-maxillomandibular ligament present; epibranchial I free, not bound to wall of pharynx by connective tissue; proximal one-half to two-thirds of ceratobranchial I bound to wall of pharynx, distal one-half to one-third free; distal end of ceratobranchial I not bound by connective tissue to adjacent ceratobranchial II; proximal one-quarter to one-half of ceratobranchials II-IV not bound together by connective tissue; pterygiophore of illicium with a small ossified rudiment of second cephalic spine; escal bulb and central lumen present, esca without tooth-like denticles; ovaries paired; pyloric caecae absent.
Metamorphosed males are further differentiated by having a series of enlarged dermal spines above and posterior to the upper denticular bone. They differ further in having the following combination of character states: eyes lateral and slightly oval in shape, with narrow aphakic space in front of lens; olfactory organs large, nostrils directed laterally; 16-31 denticular teeth on snout, 20-50 on chin, fused at base to form upper and lower denticular bones, respectively; fin-ray counts as given for metamorphosed females; skin densely covered with close-set dermal spinules; free-living, never parasitic, but temporarily attached males unknown (see Pietsch, 2005).
Larvae are differentiated from those of other ceratioids by having the following combination of character states: body short, almost spherical; skin highly inflated; pectoral fins of normal size, not reaching beyond dorsal and anal fins; pelvic fins absent; sexual dimorphism evident, females with a small, club-shaped illicial rudiment protruding from head; fin-ray counts as given for metamorphosed females; metamorphosis delayed, larvae attaining lengths of 17-22.5 mm SL, metamorphosis taking place at lengths between about 20 and 33 mm SL (Bertelsen, 1984:326, 328, fig. 169A,B; Bertelsen and Krefft, 1988:28).
Description
Metamorphosed females with body short and stout, globose, depth (in the best preserved specimens) 60-70% SL; head about 60% SL; mouth large, opening oblique to almost vertical, cleft extending below or slightly behind eye; snout and chin blunt, padded with thick skin covered with wart-like papillae, forming thick fleshy lips; lower jaw unusually thick and heavy, protruding slightly in front of upper jaw, with well-developed symphysial spine; oral valve weakly developed; nostrils set on low rounded papillae; jaw teeth relatively short, arranged in several oblique, longitudinal series, 10 to more than 20 such series in lower jaw; number of teeth on each side of upper jaw 35-150, on lower jaw 40-210; longest teeth in lower jaw 3-8% SL, those of upper jaw somewhat shorter; vomerine teeth absent; epibranchial and ceratobranchial teeth present: one or more tooth plates on proximal tips of epibranchials I-IV, 8-20 tooth plates on ceratobranchials I-IV (see Bertelsen and Krefft, 1988:16, fig. 4C); epibranchial I free from wall of pharynx, but closely bound to epibranchial II; proximal one-half to two-thirds of ceratobranchial I bound to wall of pharynx, distal one-half to one-third free; epibranchial IV and ceratobranchial IV bound to wall of pharynx, no opening behind fourth arch; gill filaments present on epibranchials II-IV, proximal one-half to two-thirds of ceratobranchial I, and full length of ceratobranchials II-IV; pseudobranch absent; illicium stout, unusually thick, length 26-86% SL; pterygiophore of illicium short, anterior end nearly completely covered by skin of head, exposed anterior tip not reaching beyond anteriormost margin of snout when protruded; escal bulb short, oval in shape, with distal light-guiding appendages; neuromasts of acoustico-lateralis system set on low rounded papillae, pattern of placement as described for other ceratioids (Pietsch, 1969, 1972, 1974a, 1974b).
Recently metamorphosed females, 30 to about 34 mm SL, differing from larger specimens in having a shorter illicium and shorter escal appendages, in lacking dermal spines and papillae on snout and chin, and in having fewer jaw teeth.

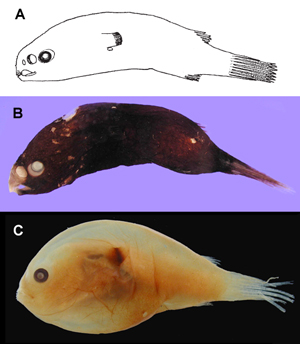
Free-living males of the family Himantolophidae. A Himantolophus sp., 34.5 mm SL (after Bertelsen, 1951, © 1951); B Himantolophus rostratus-group, MCZ 161523, 34 mm SL (© 2005 Museum of Comparative Zoology, Harvard University); C Himantolophus sp., MCZ 161024, 27 mm SL (© 2005 Museum of Comparative Zoology, Harvard University).
Metamorphosed males with eyes relatively well developed, diameter 5.5-8.7% SL; olfactory organs large, nostrils directed laterally, posterior nostril separated from eye and from anterior nostril by pigmented skin, slightly larger than anterior, greatest diameter 3.3-7.6% SL; nasal area pigmented, not inflated; olfactory lamellae 10-17; maxillae and premaxillae not reduced; jaw teeth absent; upper denticular with 16-31 recurved denticles arranged in a semicircular, fan-shaped series, with some shorter denticles within; upper denticular fused dorsally with a number of large dermal spinules of snout forming a conspicuous median ridge between large olfactory organs, and attached to anterior end of illicial pterygiophore; lower denticular with 20-50 recurved denticles more-or-less distinctly separated into a median and a pair of lateral groups (Bertelsen and Krefft, 1988:26, 75, figs. 10, 35, 36).
Larvae with skin of head and body in front of dorsal and anal fin strongly inflated and nearly spherical, with or without a dorsal pigmented hump (Bertelsen and Krefft, 1988:74, fig. 34); subdermal pigmentation with a dorsal group of melanophores well separated from peritoneal pigmentation and with a group of melanophores on caudal peduncle.
Color in preservation black to dark brown over entire surface of head and body; females of some species with whitish or lightly pigmented areas on chin, snout, and upper surface of head or body and/or with one or more shiny white patches on ventral and/or dorsal midline, at or on bases of one or more of median fins; pigmentation of illicium and fin rays increasing with size of specimens and highly variable among species.
The largest known female specimen, the holotype of H. groenlandicus from southwest Greenland, measured about 465 mm SL, and there are several additional individuals in the range of 250-400 mm SL (Bertelsen and Krefft, 1988:14, 37). The known metamorphosed males measure between 23-39 mm SL, and the largest larvae range from 17-22.5 mm SL.
Distribution
Like most ceratioid families, the Himantolophidae is widely distributed in all three major oceans of the world, with most records, including all larvae and males, confined to a broad belt limited by about 40°N and 40°S. However, large females, probably expatriates and so far all of a single species, H. groenlandicus, have been taken as far north as 65° or at the polar front in the North Atlantic.
The H. appelii-group, containing only H. appelii, appears to be restricted to the southern subtropical convergence, between approximately 27° and 43°S in all three major oceans. Likewise, each of the other species groups has a wide, probably circumglobal distribution, but besides H. appelii only two species have been recorded from more than one ocean (H. cornifer from the Atlantic and Pacific and H. macroceratoides from the Atlantic and Indian oceans). Nine species have so far been recorded only from the Atlantic Ocean. Identifiable specimens from the Indian Ocean are few: only H. appelii and H. macroceratoides mentioned above and the single known specimen of H. pseudalbinares caught slightly east of Cape Town. However, several larvae, a male, and some juvenile females caught in this ocean can be referred to the H. groenlandicus-group, and may belong to H. groenlandicus the most numerous and widely distributed Atlantic member of this group. Nine of the 12 species known from the Atlantic have been recorded from the northwestern part of this ocean and are, with the exception of H. appelii, restricted to northern waters; the remaining three species are known from too few specimens to predict their geographic range.
Key to Females of Species Groups of the Genus Himantolophus
The following key will differentiate adolescent and adult female specimens greater than approximately 33 mm SL.
1A. Posterior escal appendage present (go to 2)
1B. Posterior escal appendage absent; distal escal appendage long, 16-92% SL in specimens 30-75 mm, 32-214% in larger specimens (Himantolophus cornifer-group Bertelsen and Krefft, 1988)
2A. Distal escal appendage long (8-52% SL in specimens 30-75 mm, about 24-82% SL in larger specimens), nearly as long or longer than posterior escal appendage in specimens 30-75 mm, twice as long in larger specimens (Himantolophus albinares-group Bertelsen and Krefft, 1988)
2B. Distal escal appendage short (0.4-5% SL in specimens 30-75 mm, about 1.0-20% SL in larger specimens), distinctly shorter than posterior escal appendage (less than one-half in specimens 30-75 mm, less than two-thirds in larger specimens)(go to 3)
3A. Proximal half of distal escal appendage simple, undivided; base of escal bulb without appendages; a distal pair of appendages on illicial stem situated 1.5 to 2 times diameter of esca bulb below base of posterior escal appendage (Himantolophus nigricornis-group Bertelsen and Krefft, 1988)
3B. Distal escal appendage bifurcated at base; base of escal bulb with a distal pair of illicial appendages, less than diameter of escal bulb below base of posterior escal appendage (go to 4)
4A. Posterior escal appendage bifurcated at base, each primary branch with an anterior series of 2-7 side-branches; distal swellings of escal bulb not distinctly divided into four lobes (Himantolophus appelii-group Bertelsen and Krefft, 1988)
4B. Posterior escal appendage with (1) proximal part undivided and distal part simple or bifurcated, or (2) divided near base to form three simple filaments, or (3) represented by two or three separate undivided filaments; base of distal escal appendage surrounded by four well-developed escal lobes (Himantolophus groenlandicus-group Bertelsen and Krefft, 1988)
References
Bertelsen, E. 1951. The ceratioid fishes. Ontogeny, taxonomy, distribution and biology. Dana Rept., 39, 276 pp.
Bertelsen, E., T. W. Pietsch, and R. J. Lavenberg. 1981. Ceratioid anglerfishes of the family Gigantactinidae: Morphology, systematics, and distribution. Nat. Hist. Mus. L. A. Co., Contri. Sci., 332, vi + 74 pp.
Bertelsen, E. 1984. Ceratioidei: Development and relationships. pp. 325-334, In: Moser, H. G., W. J. Richards, D. M. Cohen, M. P. Fahay, A. W. Kendall, Jr., and S. L. Richardson (editors), Ontogeny and Systematics of Fishes, Spec. Publ. No. 1, Amer. Soc. Ichthy. Herpet., ix + 760 pp.
Bertelsen, E., and G. Kreft. 1988. The ceratioid family Himantolophidae (Pisces, Lophiiformes). Steenstrupia, 14(2): 9–89.
Pietsch, T. W. 1969. A remarkable new genus and species of deep-sea anglerfish (family Oneirodidae) from off Guadalupe Island, Mexico. Copeia, 1969(2): 365–369.
T. W. 1972. A second specimen of the deep-sea anglerfish, Phyllorhinichthys micractis (family Oneirodidae), with a histological description of the snout flaps. Copeia, 1972(2): 335–340.
Pietsch, T. W. 1974a. Osteology and relationships of ceratioid anglerfishes of the family Oneirodidae, with a review of the genus Oneirodes Lütken. Nat. Hist. Mus. L. A. Co., Sci. Bull., 18, 113 pp.
Pietsch, T. W. 1974b. Systematics and distribution of ceratioid anglerfishes of the genus Lophodolos (family Oneirodidae). Breviora, 425: 1–19.
Pietsch, T. W. 1975. Systematics and distribution of ceratioid anglerfishes of the genus Chaenophryne (family Oneirodidae). Bull. Mus. Comp. Zool., 147(2): 75-100.
Pietsch, T. W. 2005. Dimorphism, parasitism, and sex revisited: modes of reproduction among deep-sea ceratioid anglerfishes (Teleostei: Lophiiformes). Ichthyol. Res., 52: 207–236.
Title Illustrations

| Scientific Name | Himantolophus albinares Maul |
|---|---|
| Location | Western North Atlantic, south of Hudson Canyon, 39°5'N, 72°27'W, 992-1098 m |
| Specimen Condition | Dead Specimen |
| Identified By | K. E. Hartel |
| Sex | Female |
| Life Cycle Stage | Adult |
| Size | 100 mm SL |
| Collection | Museum of Comparative Zoology |
| Collector | K. E. Hartel aboard F/V Contender |
| Copyright | © 2005 Museum of Comparative Zoology, Harvard University |
About This Page
Theodore W. Pietsch

University of Washington, Seattle, Washington, USA
Correspondence regarding this page should be directed to Theodore W. Pietsch at and Christopher P. Kenaley at
Page copyright © 2005 Theodore W. Pietsch
 Page: Tree of Life
Himantolophidae. Himantolophus. Footballfishes.
Authored by
Theodore W. Pietsch.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Himantolophidae. Himantolophus. Footballfishes.
Authored by
Theodore W. Pietsch.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
- First online 05 November 2005
- Content changed 02 October 2007
Citing this page:
Pietsch, Theodore W. 2007. Himantolophidae. Himantolophus. Footballfishes. Version 02 October 2007 (under construction). http://tolweb.org/Himantolophus/22004/2007.10.02 in The Tree of Life Web Project, http://tolweb.org/







 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site