Kinetoplastida
Julius Lukes

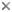
This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxRelationships after Simpson et al. 2006.
Introduction
Kinetoplastids (Kinetoplastea) are a widespread and very important group of obligatory parasitic protists. At least one stage in the life cycle of all members of this group is represented by a slender and highly flexible cell equipped with one or two flagella, arising from a prominent flagellar pocket. Another hallmark is the presence of extensive mitochondrial DNA, termed kinetoplast DNA and trans-splicing. Usually a centrally located nucleus can be seen in Giemsa-stained smeared cells. The size of the kinetoplastid cell varies from about 10 to 100 microns in length, and never exceeds 20 microns in width. When present in the life cycle, the intracellular stage is usually round and lacks the flagellum.
Since there are few morphological traits visible by light microscopy, electron microscopy is needed for visualization of subcellular structures. The flagellum is supported by a prominent structure, characteristic for kinetoplastid flagellates, termed paraflagellar rod. It is always well visible in cross-sectioned flagella with the exception of the part of the flagellum within the flagellar pocket. Cells are bound by a typical pellicular membrane, below which a series of evenly spaced subpellicular microtubules is located.
Kinetoplastid cells also contain a full set of typical eukaryotic organelles, such as a usually oval nucleus with a single central nucleolus, Golgi apparatus and endoplasmic reticulum. The size and shape of the mitochondrion, as well as the number of mitochondrial cristae are highly variable depending on the life cycle stage.
Discussion of Phylogenetic Relationships
On the grounds of morphology, kinetoplastids have been classified into two monophyletic groups, the biflagellate bodonids and uniflagellate trypanosomatids (Vickerman 1974). Molecular phylogeny confirmed the impression from ultrastructural analyses, namely that bodonids are a much more diverse group than the facultatively parasitic and morphologically rather uniform trypanosomatids. Furthermore, the descendence of trypanosomatids from bodonids is strongly supported by the molecular data. Parasitism apparently arose independently multiple times. Based solely on molecular data, two kinetoplastids are only very distantly related to the rest of the group, namely Ichthyobodo and Perkinsiella, for which a subclass Prokinetoplastina has been established (Moreira et al., 2004; Simpson et al., 2006).
References
Benne, R., J. Van den Burg, J.P.J. Brakenhoff, P. Sloof , J.H. Van Boom and M.C. Tromp. 1986. Major transcript of the frameshifted coxII gene from trypanosome mitochondria contains 4 nucleotides that are not encoded in the DNA. Cell 46: 819-826.
Berriman M., E. Ghedin and C. Hertz-Fowler. 2005. The genome of the African trypanosome Trypanosoma brucei. Science 309: 416-422.
Breitling R., S. Klinger and N. Callewaert. 2002. Non-pathogenic trypanosomatid protozoa as a platform for protein research and production. Protein Expr. Purif. 25: 209-218.
Brugerolle G., J. Lom, E. Nohynkova and L. Joyon. 1979. Comparaison et evolution des structures cellulaires chez plusieurs especes de Bodonides et Cryptobiides apprtenant aux genres Bodo, Cryptobia et Trypanoplasma (Kinetoplastida, Mastigophora). Protistologica 15: 197-221.
Brun R. and O. Balmer. 2006. New development in human African trypanosomiasis. Curr. Opinion Infect. Dis. 19: 415-420.
Campbell D.A., S. Thomas and N.R. Sturm. 2003. Transcription in kinetoplastid protozoa: why be normal. Microbes and Infection 5: 1231-1240.
Cross G.A.M. 2003. Antigenic variation in African trypanosomes and malaria. In: Molecular Medical Parasitology (edited by Marr J.J., Nilsen T.W. & Komuniecki R.W.).
De Souza W. 2007. Chagas disease: facts and reality. Microbes Infect. 9: 544-545.
De Souza W. and M.C.M. Motta. 1999. Endosymbiosis in protozoa of the Trypanosomatidae family. FEMS Microbiol. Lett. 173: 1-8.
Dujardin J.C. 2006. Risk factors in the spread of leishmanises: towards integrated monitoring? Trends Parasitol. 22: 4-6.
Dyková I., I. Fiala, J. Lom and J. Lukeš. 2003. Perkinsiella amoebae-like endosymbionts of Neoparamoeba spp., relatives of the kinetoplastid Ichthyobodo. Europ. J. Protistol. 39: 37-52.
Gadelha C., B. Wickstead, W. de Souza, K. Gull and N. Cunha-e-Silva. 2005. Cryptic paraflagellar rod in endosymbiont-containing kinetoplastid protozoa. Eukaryot. Cell 4: 516-525.
Gunzl A. 2003. Transcription. In: Molecular Medical Parasitology (edited by Marr J.J., Nilsen T.W. & Komuniecki R.W.).
Lai D.H., H. Hashimi, Z. Lun, F.J. Ayala and J. Lukeš. 2008. Adaptation of Trypanosoma brucei to gradual loss of kinetoplast DNA: T. equiperdum and T. evansi are petite mutants of T. brucei. Proc. Natl. Acad. Sci. USA 105: 1999-2004.
Lukeš J., D.L. Guilbride, J. Votypka, A. Zikova, R. Benne and P.T. Englund. 2002. The kinetoplast DNA network: Evolution of an improbable structure. Eukaryot. Cell 1: 495-502.
Lukeš J., H. Hashimi and A. Zikova. 2005. Unexplained complexity of the mitochondrial genome and transcriptome in kinetoplastid flagellates. Curr. Genet. 48: 277-299.
Lukeš J., I.L. Mauricio, G. Schönian, J.C. Dujardin, K. Soteriadou, J.P. Dedet, K. Kuhls, W. Quispe Tintaya, M. Jirků, E. Chocholová, C. Haralambous, F. Pratlong, M. Oborník, A. Horák, F.J. Ayala and M.A. Miles. 2007. Evolutionary and geographical history of the Leishmania donovani complex with a revision of current taxonomy. Proc. Natl. Acad. Sci. USA 104: 9375-9380.
Maslov D.A. and L. Simpson. 1994. RNA editing and mitochondrial genomic organization in the cryptobiid kinetoplastid protozoan Trypanoplasma borreli. Mol. Cell. Biol. 14: 8174-8182.
Moreira D., P. Lopez-Garcia and K. Vickerman. 2004. An updated view of kinetoplastid phylogeny using environmental sequences and a closer outgroup: proposal for a new classification of the class Kinetoplastea. Int. J. Syst. Evol. Microbiol. 54: 1861-1875.
Schonian G., A. Nasereddin, N. Dinse, C. Schweynoch, H.D.F.H Schallig, W. Presber and C.L. Jaffe. 2003. PCR diagnosis and characterization of Leishmania in local and imported clinical samples. Diagn. Microbiol. Infect. Dis. 47: 349-358.
Simpson A.G.B., J.R. Stevens and J. Lukeš. 2006. The evolution and diversity of kinetoplastid flagellates. Trends Parasitol. 22: 168-174.
Stuart K., R. Brun, S. Croft, A. Fairlamb, R.E. Gurtler, J. McKerrow, S. Reed and Tarleton, R. 2008. Kinetoplastids: related protozoan pathogens, different diseases. J. Clin. Invest. 118: 1301-1310.
Vickerman K. 1974. The ultrastructure of pathogenic flagellates. Trypanosomiasis and Leishmaniasis. Elsevier.
Westenberger S.J., C. Barnabe, D.A. Camnpbell and N.R. Sturm. 2005. Two hybridization events define the population structure of Trypanosoma cruzi. Genetics 171: 527-543.
Yurchenko V., J. Lukeš, X. Xu and D.A. Maslov. 2006. An integrated morphological and molecular approach to a new species description in the Trypanosomatidae: the case of Leptomonas podlipaevi n.sp., a parasite of Boisea rubrolineata (Hemiptera: Rhopalidae). J. Euk. Microbiol. 53: 103-111.
Yurchenko V.A., J. Lukeš, M. Tesařová, M. Jirků and D.A. Maslov. 2008. Morphological discordance of the new trypanosomatid species phylogenetically associated with the genus Crithidia. Protist 159: 99-114.
Title Illustrations

| Scientific Name | Trypanosoma brucei |
|---|---|
| Identified By | Jan Votypka |
| Copyright |
© 2008 Jan Votýpka

|
About This Page
This page is being developed as part of the Tree of Life Web Project Protist Diversity Workshop, co-sponsored by the Canadian Institute for Advanced Research (CIFAR) program in Integrated Microbial Biodiversity and the Tula Foundation.

University of South Bohemia in Ceske Budejovice, Czech Republic
Correspondence regarding this page should be directed to Julius Lukes at
Page copyright © 2009
All Rights Reserved.
- First online 02 January 2009
- Content changed 02 January 2009
Citing this page:
Lukes, Julius. 2009. Kinetoplastida. Version 02 January 2009 (under construction). http://tolweb.org/Kinetoplastida/98013/2009.01.02 in The Tree of Life Web Project, http://tolweb.org/






 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site