Leotiomycetes
Zheng Wang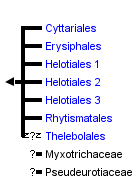

This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
The Leotiomycetes includes non-lichenized fungi producing a generally small apothecium with an exposed hymenium and an inoperculate, unitunicate ascus that has an apical perforation pore for releasing ascospores (Dennis 1968, Korf 1973, Nannfeldt 1932, Pfister and Kimbrough 2001). Recent molecular studies suggest that several groups of fungi with simple, cleistothecial ascomata belong to the Leotiomycetes, including the Erysiphales, Myxotrichaceae, and Thelebolales (Blackwell et al. 2006; de Hoog et al. 2005; Gernandt et al. 2001). Conversely, other groups traditionally included in the Leotiomycetes, such as the Geoglossaceae and Orbiliaceae, have been proved to be phylogenetically distinct (Spatafora et al. 2006; Wang et al. 2006). Currently, five orders, 21 families, and about 510 genera (115 with an uncertain position) are accepted in the Leotiomycetes on the basis of both traditional classification and molecular phylogenetic studies (Eriksson 2005, Kirk et al. 2001, Hibbett et al. 2007).
Morphologically and ecologically, the Leotiomycetes is a highly diverse group of the Pezizomycotina. For example, species of Cyttaria (Cyttariales) produce brightly colored globose fruiting bodies of "ping-pong ball" size on hard wood trees, while apothecia of Lophodermium (Rhytismatales) often mature as small, dark dots on conifer needles. The Erysiphales and Thelebolales are associated with distinct ecological characters and nutritional modes, in addition to morphology almost unique for each group. Members of the Helotiales thrive in various ecosystems and cover a broad range of niches, and helotialean fungi have been described as plant pathogens, endophytes, mycorrhizae, fungal parasites, terrestrial saprobes, aquatic saprobes, root symbionts, and wood rot fungi.


.200a.jpg)


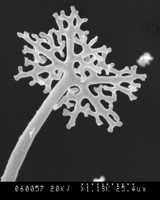
Top left and center: SEM (scanning electron micrographs) of Microsphaera palczewskii Jacz. (Erysiphales) showing appendages branching dichotomously multiple times from cleistothecia. Plant Disease 87: 451. © . Top right: Aero-aquatic fungus Mitrula elegans (Heliothales) Berk. © Michael Wood. Bottom left: Chlorociboria aeruginascens (Nylander) Kanouse from Viry, Haute-Savoie, France. © . Bottom right: Wood decay caused by Chlorociboria aeruginascens © Gary Emberger
Many species of the Leotiomycetes are described from the temperate Northern Hemisphere, but some members within the Leotiomycetes are patchy in their broad geographic distribution. For example many fungi of the Sclerotiniaceae (Helotiales) are plant pathogens, and these fungi might have evolved primarily in the Northern Hemisphere. However, other genera, such as Cyttaria spp. and Chlorovibrissea, are only found in the Southern Hemisphere.



Two fungi only known from the Southern Hemisphere (from left to right): Cyttaria gunnii; Chlorovibrissea phialophora.© Clive Shivley
Characteristics
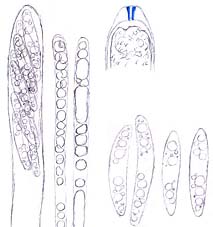
Ascus (showing an apical perforation pore with the wall turning blue in iodine solution), paraphyses and ascospores of Mollisia ventosa. © Hans-Otto Baral
Asci of leotiomyceteous fungi and some other fungi (e.g. Sordariomycetes) are usually thin- and single-walled (unitunicate) with an apical perforation pore (inoperculate ascus) instead of a delicate lid-like apparatus (operculate ascus as that of Peziza species) for releasing ascospores. The traditional concept of the Leotiomycetes includes only fungi producing an apothecium which opens at a very early development stage with the hymenium exposed, so leotiomyceteous fungi are sometimes called inoperculate discomycetes (fungi with an apothecium) as well. The current concept of the Leotiomycetes includes fungal groups very different from each other in morphology. Some of them produce an ascocarp with the hymenium enclosed all the time or until the very late stage of their development, and this makes it difficult to integrate members of the class using just some key characters and to give a meaningful common name to the group. However, fungi of each order in the Leotiomycetes generally share a unique macromorphology, anatomic structure, and ecological role.
Cyttariales: There is a single family, Cyttariaceae, with a single genus, Cyttaria, of about a dozen parasitic species on southern beech (Nothofagus) in the Southern Hemisphere. The fungus produces large and fleshy globose or pyriform stromata with numerous separate, cup-shaped chambers containing asci and dark ascospores on galls of living branches. Different authors have used morphological evidence to place the genus variously in the Pezizales and the Helotiales (see discussion in Gamundí 1991). Megoni (1986) showed that the asci have a thin cell wall and an apical amyloid ring, characters shared by some helotialean fungi.
Erysiphales: The Erysiphales with one family of 15 genera (Braun et al. 2002, Takamatsu et al. 2005a, b) are obligate plant pathogens that cause powdery mildew (mitotic stages of these fungi can cover the host plant and give the infected area a unique, white-powder appearance) diseases on about 10,000 plant species (Amano 1986). There are no direct morphological features supporting inclusion of these fungi in the Leotiomycetes, and without exception, all Erysiphales have closed, non-ostiolate fruit-bodies with persistent asci and basal hymenium.
Rhytismatales: Many of the taxa placed in the Rhytismatales are poorly understood, and probably most fungi in this order have an endophytic life-style living harmlessly inside host plants. For most species the teleomorph comprises an apothecium with a very reduced excipulum, which develops within a dark stroma, and the stroma is typically immersed within host tissue and sometimes covered by a clypeus (Johnston 2001; Wang et al. 2002). The hymenium becomes exposed after the covering host and fungal tissue splits by one or more elongate slits. The asci are typically thin-walled, undifferentiated at the apex, nonamyloid, ascospores typically with gelatinous sheaths or appendages. The anamorphs are mostly Leptostroma-like, the spermatial conidia developing on often percurrently proliferating conidiogenous cells.
Thelebolales: Species of Thelebolus produce small, globose to disk-shaped ascomata with sometimes polysporus asci (ascus with more than 8 spores), and the development of the ascomata varies from a cleistohymenial type to a eugymnohymenial type (van Brummelen 1967). The Thelebolaceae has been placed in the Pezizales (Eckblad 1968, Kimbrough and Korf 1967), and this placement has been studied from the apothecium macro-morphology to ascus ultrastructure (Brummelen 1998). The placement of the Thelebolales in the Leotiomycetes is based on SSU-rDNA sequence data, and many of the order have still not been included in molecular phylogenetic studies (Gernandt et al. 2001, Landvik et al. 1998, Momol et al. 1996, de Hoog et al. 2005). Some species are coprophilous (on dung), and their sexual stage and prominent polysporus asci can be so easily induced in vitro that they have been used to demonstrate sexual development of ascomyceteous fungi.


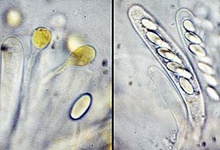


From left to right: ascocarps and paraphyses and an ascospore, and 8-spore asci of Thelebolus microsporus; ascocarps, and a multi-spore ascus of T. stercoreus. © Jens H. Peterson

Apothecia of Dumontinia tuberosa (Helotiales) from stromatized (blackened) plant tissue. © Jaroslav Maly
Helotiales (with exclusion of the Geoglossaceae): As the largest order of the inoperculate discomycetes, helotialean fungi are characterized morphologically by an apothecium (cup- or disc-shaped) with an exposed hymenium and thin-walled, eight ascospores ascus with slightly thickened apices. Except for some famous plant pathogens, such as Sclerotinia sclerotiorum (also known as white mold) and Fabrella tsugae (also known as needle blight of Hemlock), life styles of most fungi in this order are unknown. Many members in the Helotiales were recorded as saprobic, but this requires much careful investigation. Especially if species have been recorded as host-restricted saprobes, often this relationship is an indication of an endophytic stage in their life cycle (Wang et al. 2006a, b).
Although fungi in the Leotiomycetes are morphologically diverse, as you can see from photographs of some representatives here, morphological characters used for taxonomy and classification of this class are very limited. We should admit that we have not done enough detailed studies about their morphology rather than saying these fungi are morphologically simple, and we just start to understand how ecological and biological factors play a role in shaping the evolutionary history and appearances of these interesting fungi.




Three related fungi in the Hemiphacidiaceae (Helotiales) show significant morphological differences associated with different lifestyles (from left to right): needle blight of hemlock–Fabrella tsugae producing tiny apothecia as dark-colored dots on living trees. © PA-DCNR,Forestry Archives; endophyte–Heyderia abietis producing small apothecia on fallen spruce needles. © Jens H. Petersen; saprobic fungus–Chlorencoelia versiformis produces medium-size apothecia on rotten wood. ©

The asexual stage (producing white conidiospores) and sexual stage (disc-like apothecia) happen simultaneously in Holwaya mucida. © Jaroslav Maly
Like most other ascomycetes, members of the Leotiomycetes have a predominantly asexual life cycle, via which large amounts of conidiospores (mitotic spores) are produced, and this character is critical for fungi, especially pathogens, to compete with other organisms for resources. But the life history of few leotiomyceteous fungi has been studied, and there are many leotiomyceteous fungi known only with either sexual (teleomorph) or asexual stages (anamorph). Anamorphs in various environmental samples, including some root endophytes and aquatic hyphomycetes, have been suggested to belong in the Leotiomycetes, but without any clear teleomorph connections. To make studies of this class even more difficult, there is little correlation between the classifications of teleomorphs and anamorphs in the Leotiomycetes (Marvanova 1997; Sutton and Hennebert 1994; also see Freshwater Ascomycetes and their Anamorphs).
Discussion of Phylogenetic Relationships
The Leotiomycetes represents a morphologically and ecologically diverse class of Pezizomycotina whose evolutionary history is only beginning to be unraveled through phylogenetic analyses of molecular data. The traditional view of relationships within the Leotiomycetes has experienced significant changes. With very limited sampling, the Leotiomycetes (Eriksson 2005; Hibbett et al. 2007) excluding the Geoglossaceae and including the Pseudeurotiaceae is supported as a monophyletic group by rDNA data and protein coding gene data (Spatafora et al. 2006; Wang et al. 2006). Here we accept the Cyttariales, Erysiphales, Helotiales, Rhytismatales, Thelebolales, Myxotrichaceae and Pseudeurotiaceae as members of the Leotiomycetes based on analyses by the Assembling the Fungal Tree of Life (AFTOL) project and other previously published molecular phylogenies.
The placement of the Thelebolales in the Leotiomycetes is based on SSU-rDNA sequence data, and many representatives of this order have still not been included in molecular phylogenetic studies (e.g. Gernandt et al. 2001, Landvik et al. 1998, Momol et al. 1996). The Thelebolales may not be monophyletic, and its position in the Leotiomycetes should be regarded as a temporary treatment until sufficient proof becomes available. Myxotrichaceae and Pseudeurotiaceae are two families of cleistothecial ascomycetes, both of which contain genera linked to the Leotiomycetes in molecular studies (Sogonov et al. 2005, Sugiyama et al. 1999, Suh and Blackwell 1999). The molecular-based phylogenies are supported by electron microscope studies, with the highly reduced cleistothecial ascomata of Myxotrichum showing a striking similarity in morphogenesis and gross morphology to typical helotialean fungi such as Hymenoscyphus species (Tsuneda and Currah 2004). Inclusion of Pseudeurotiaceae in the Leotiomycetes is tentative, however, analysis of protein coding data of Pseudeurotium also supported its affinity to Leotia (Spatafora et al. 2006).
Within the Leotiomycetes, the Cyttariales, Rhytismatales and Erysiphales of current definition have been supported as monophyletic with both morphological and molecular characters. The current concept of the Helotiales (Eriksson 2005) almost certainly includes non-monophyletic taxa, and the Helotiales has received more attention recently (e.g. Gernandt et al. 2001, Lutzoni et al. 2004, Wang et al. 2005). Even with exclusion of groups such as the Cyttariales, Erysiphales, Myxotrichaceae, Pseudeurotiaceae, Geoglossaceae and genetically widely divergent taxa such as Chlorociboria and Neobulgaria species, the monophyly of the Helotiales is not supported by rDNA data, and relationships among major helotialean clades are uncertain. Relationships among major groups in the Leotiomycetes (equal to backbones of all published Leotiomycetes trees) were not resolved at all. However, several small and world-wide distributed genera, such as Ascocoryne, Bisporella, Bulgaria, Chlorociboria, Cordierites, Leotia and Microglossum, might be the key taxa for understanding the evolutionary history of the Leotiomycetes. Unfortunately molecular data for these fungi from different geographic regions are not available.



.150a.jpg)
Some species of world-wide distributed helotialean genera (from left to right): Ascocoryne cylichnium. © Jens H. Petersen; Bulgaria inquinans. © Christian Pourre; Microglossum viride. © Michael Wood.
Significant advancements have been made in the phylogeny of the Leotiomycetes and include: the classification of Cudoniaceae in the Rhytismatales; the inclusion of the Erysiphales, Cyttariales, and Pseudeurotiaceae in the Leotiomycetes; and the exclusion of Geoglossaceae from the Leotiomycetes. Current taxon and character sampling is insufficient to address many of the internal nodes of the class, and future phylogenetic studies must strive to significantly increase character sampling, especially that of protein-coding genes, from the diversity of species characterized as inoperculate discomycetes
References
Amano K. 1986. Host Range and Geographical Distribution of the Powdery Mildew Fungi. Japan Scientific Societies Press, Tokyo, Japan.
Blackwell M, Hibbett DS, Taylor JW, Spatafora JW. 2006 Research coordination networks: a phylogeny for kingdom fungi (deephypha). Mycologia 98: 829-837.
Braun U. 1981. Taxonomic studies in the genus Erysiphe I. Generic delimitation and position in the system of the Erysiphaceae. Nova Hedwigia 34:679-719.
Brummelen J van. 1967. A world-monograph of the genera Ascobolus and Saccobolus (Ascomycetes, Pezizales). Persoonia (Suppl.) 1: 1-260.
Brummelen J van. 1998. Reconsideration of relationships within the Thelebolaceae based on ascus ultrastructure. Persoonia 16: 425-469.
de Hoog GS, Göttlich E, Platas G, Genilloud O, Leotta G, van Brummelen J. 2005. Evolution, taxonomy and ecology of the genus Thelebolus in Antarctica. Stud Mycol 51: 33-76.
Dennis RWG. 1968. British Ascomycetes. Cramer, Lehre.
Eckblad FE. 1968. The genera of the operculate discomycetes. A re-evaluation of their taxonomy, phylogeny and nomenclature. Norw J Bot 15: 1-191.
Eriksson OE. 2005. Outline of Ascomycota - 2005. Myconet 11: 1–113.
Gamundí IJ. 1991. Review of recent advances in the knowledge of the Cyttariales. Syst Ascomycet 10: 69-77.
Gernandt DS, Platt JL, Spatafora JW, Holst-Jensen A, Hamelin RC, Kohn LM. 2001. Phylogenetics of Helotiales and Rhytismatales based on partial small subunit nuclear ribosomal DNA sequences. Mycologia 93: 915-933.
Hibbett DS, Binder M, Bischoff JF and 64 co-authors. 2007. A higher-level phylogenetic classification of the Fungi. Mycological Research 111: 509-547.
Johnston PR. 2001. Monograph of the monocotyledon-inhabiting species of Lophodermium. Mycol Papers 176: 1–239.
Kirk PM, Cannon PF, David JC, Stalpers JA. 2001. Ainsworth and Bisby’s dictionary of the fungi, 9th edition. CAB International, Wallingford.
Korf RP. 1973. Discomycetes and Tuberales. In Ainsworth GC, Sparrow FK, Sussman AS (des). The Fungi: an advanced treatise, vol IVA. Academic Press, Now York, pp 249-319.
Landvik S, Kristiansen R, Schumacher T. 1998. Phylogenetic and structural studies in the Thelebolaceae (Ascomycota). Mycoscience 39: 49-56.
Lutzoni F, Kauff F, Cox CJ, and 43 co-authors. 2004. Where are we in assembling the fungal tree of life, classifying the fungi, and understanding the evolution of their subcellular traits. Am J Bot 91: 1446-1480.
Marvanova L. 1997. Freshwater hyphomycetes: a survey with remarks on tropical taxa. In: Janardhanan, K.K., Rajendran, C., Natarajan, K., Hawksworth, D.L. (Eds.), Tropical Mycology. Science Publishers, Inc., USA, pp. 169-226.
Momol EA, Kimbrough JW, Eriksson OE. 1996. Phylogenetic relationships indicated by 18S rDNA sequence analysis. Syst Ascomycet 14: 91-100.
Nannfeldt JA. 1932. Studien über die Morphologie und Systematik der nichtlichenisierten inoperculaten Discomyceten. Nova Acta R Soc scient upsal 8: 1-368.
Pfister DH, Kimbrough JW. 2001. Discomycetes. In: McLaughlin, D.J., McLaughlin, E.G., Lemke, P. A. (Eds), The Mycota VII Part A. Systematics and Evolution. Springer-Verlag, Berlin Heidelberg, pp. 257-281.
Sogonov MV, Schroers HJ, Gams W, Dijksterhuis J, Summerbell RC. 2005. The hyphomycete Teberdinia hygrophila gen. nov. and related anamorphs of Pseudeurotium species. Mycologia 97: 695-709.
Spatafora JW, Sung G-H, Johnson D, Hesse C, and 32 co-authors. 2006. A five-gene phylogeny of Pezizomycotina. Mycologia 98: 1018-1028.
Sutton BC, Hennebert GL. 1994. Interconnections amongst anamorphs and their possible contribution to ascomycete systematics. In: D. L. Hawksworth (Ed.), Ascomycete systematics: problems and perspectives in the nineties. Plenum Press, New York, pp. 77-100.
Sugiyama M, Mikawa T. 2001. Phylogenetic analysis of the non-pathogenic genus Spiromastix (Onygenaceae) and related onygenalean taxa based on large subunit ribosomal DNA sequences. Mycoscience 42: 413-421.
Suh SO, Blackwell M. 1999. Molecular phylogeny of the cleistothecial fungi placed in Cephalothecaceae and Pseudeurotiaceae. Mycologia 91: 836-848.
Takamatsu S, Braun U, Limkaisang S. 2005a. Phylogenetic relationships and generic affinity of Uncinula septate inferred from nuclear rDNA sequences. Mycoscience 46: 9-16.
Takamatsu S, Niinomi S, De Álvarez MGC, Álvarez RE, Havrylenko M, Braun U. 2005b. Caespitotheca gen. nov., an ancestral genus in the Erysiphales. Mycol Res 109: 903-911.
Tsuneda A, Currah RS. 2004. Ascomatal morphogenesis in Myxotrichum arcticum supports the derivation of the Myxotrichaceae from a discomycetous ancestor. Mycologia 96: 627-635.
Wang Z, Binder M, Hibbett DS. 2002. A new species of Cudonia based on morphological and molecular data. Mycologia 94: 641-450.
Wang Z, Binder M, Hibbett DS. 2005. Life history and systematics of the aquatic discomycetes Mitrula (Helotiales, Ascomycota) based on cultural, morphological, and molecular studies. Am J Bot 92: 1565-1574.
Wang Z, Binder M, Schoch CL, Johnston PR, Spatafora JW, Hibbett DS. 2006a. Evolution of helotialean fungi (Leotiomycetes, Pezizomycotina): a nuclear rDNA phylogeny. Mol Phylogenet Evol 41:295-312.
Wang Z, Johnston PR, Takamatsu S, Spatafora JW, Hibbett DS. 2006b. Toward a phylogenetic classification of the Leotiomycetes based on rDNA data. Mycologia 98: 1065-1075.
Information on the Internet
- MSA. Official website of Mycological Society of America.
- IMA. Official website of International Mycological Associatioin.
- Myconet . The present classification contains all accepted genera and higher taxa above the generic level in phylum Ascomycota.
- Macrofungi in Chile. A nice webpage about fungi from South America.
- Mycokey. An excellent website for studying ascomycetes with a lot of nice photographs and illustrations.
- Mykoweb. Many nice photographs of California fungi.
- New Zealand fungi 1. Fungi collected from New Zealand.
- Hiddenforest. Many beautiful photos of New Zealand’s fungi.
- Aquatic hyphomycetes. Information of many aquatic fungi and their anamorphs.
- Czech fungi. Nice pictures of fungi from Czech Republic.
- Découvertes Mycologiques! Nice pictures of fungi from France.
- Haute-Savoie Champignons. Nice pictures of fungi from Europe.
- CABI database. Very useful website for all kinds of information.
Title Illustrations

| Scientific Name | Cyttaria espinosae |
|---|---|
| Location | Malleco Forest Reserve, Chile |
| Identified By | Goetz Palfner |
| Copyright | © Goetz Palfner |
| Scientific Name | Rhytisma americanum Hudler & Banik |
|---|---|
| Location | Roosevelt State Park, New York State, USA |
| Creator | Photographed by Kent E. Loeffler |
| Specimen Condition | Live Specimen |
| Identified By | G. W. Hudler |
| Type | Holotype |
| Collector | G. W. Hudler |
| Copyright | © |
| Scientific Name | Leotia lubrica |
|---|---|
| Copyright | © Michael Wood |
About This Page
Zheng Wang

Yale University
Correspondence regarding this page should be directed to Zheng Wang at
Page copyright © 2007 Zheng Wang
 Page: Tree of Life
Leotiomycetes.
Authored by
Zheng Wang.
The TEXT of this page is licensed under the
Creative Commons Attribution License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Leotiomycetes.
Authored by
Zheng Wang.
The TEXT of this page is licensed under the
Creative Commons Attribution License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
- First online 19 March 2007
- Content changed 26 September 2007
Citing this page:
Wang, Zheng. 2007. Leotiomycetes. Version 26 September 2007. http://tolweb.org/Leotiomycetes/29048/2007.09.26 in The Tree of Life Web Project, http://tolweb.org/











 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site