Hynobiidae
Allan Larson, David Wake, and Tom Devitt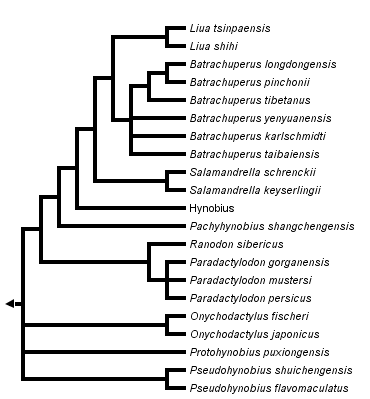


This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxPhylogenetic relationships among living hynobiid salamanders inferred from complete mitochondrial genomic DNA sequences (Zhang et al., 2006).
Introduction
Hynobiids are small to medium sized (up to 200 mm in Ranodon) salamanders found primarily in Asia with one species distributed in European Russia (AmphibiaWeb: Hynobiidae). These salamanders have a biphasic life cycle with aquatic larvae and metamorphosed adults that are terrestrial or aquatic. Larvae have external gills, four pairs of gill slits and caudal fins. Metamorphosed adults are terrestrial during nonreproductive periods, except for the genera Batrachuperus, Liua, and Pachyhynobius, which live in water (Zhao et al., 1988). Metamorphosed adults have well-developed lungs except for the genus Onychodactylus, which is lungless. Hynobiids have external fertilization, an angular bone in the lower jaw, and large numbers of microchromosomes; these traits are considered ancestral for salamanders and have caused some authors to postulate that hynobiids resemble the most recent common ancestor of all salamanders more closely than do salamanders of the other families (Hecht and Edwards, 1977).
Hynobiids comprise the only family of salamanders whose current geographic distribution is entirely Asian. They are easily distinguished from other Asian salamanders which include rough-skinned newts (family Salamandridae) and giant, nonmetamorphic salamanders of the family Cryptobranchidae.
Eggs may be deposited in streams or ponds, varying among species. Eggs are laid in arc-shaped, gelatinous sacs and attached to stones or vegetation in the water, where they are fertilized. The number, size and arrangement of eggs in the gelatinous sacs vary among genera and are used as taxonomic characters for examining intergeneric relationships (Zhao et al., 1988).
Characteristics
Diagnosis
Hynobiids are moderate-sized to small salamanders and have a biphasic life cycle with aquatic larvae and metamorphosed adults. While there are no derived morphological features for this group, all hynobiids exhibit the following characters: 1) septomaxillae present; 2) lacrimal present; 3) vomerine teeth that are not parallel to marginal teeth row; and 4) ribs unicapitate (www.amphibiaweb.org). Larvae have external gills, four pairs of gill slits, and a caudal fin; all of these characteristics are lost at metamorphosis, and eyelids are acquired. Eggs are laid in arc-shaped, gelatinous masses in water and their fertilization is external. Lungs are present and well developed except in the genus Onychodactylus, which has no lungs.
Detailed Characteristics of the Hynobiidae
The morphological characters given below are the ones standardly used to diagnose the salamander family Hyobiidae and to assess its phylogenetic relationships to other salamanders. The individual characteristics in most cases are shared with other salamanders and should not be interpreted as synapomorphies of the Hynobiidae. Absence of characteristics found in other salamanders is noted where it is important for distinguishing hynobiids from other salamanders and/or determining their relationships to other salamanders. These characteristics were assembled from a large number of original sources by Duellman and Trueb (1986), Larson (1991) and Larson and Dimmick (1993).
Morphology of the Skull
The premaxilla may consist of separated, paired bones, or these bones may be fused to form a single premaxillary bone. Bilaterally paired nasal bones each ossify from 2 anlagen, one positioned medially and the other laterally on the skull; the paired nasal bones abut each other, partially bisected by short posterior processes of the premaxillae. Maxillary bones are present and usually well developed. Bilaterally paired septomaxillary bones are present. Lacrimal bone is present. Quadratojugal bone is absent. Pterygoid bone is absent. Internal carotid foramina are absent from parasphenoid bones. The angular bone is separated from the mandible. The columella and operculum appear as separate ear bones detached from the otic capsule in some species, whereas others lack the operculum. Replacement of vomerine teeth proceeds from the posterior of the vomer. Teeth have a distinct crown and pedicel. Origin of the levator mandibulae anterior superficialis muscle includes the exoccipital.
Inner ear
A basilaris complex is present in the inner ear. The recessus amphibiorum is oriented horizontally in the inner ear. The otic sac is bulbar and unvascularized. The amphibian periotic canal lacks fibrous connective tissue. The periotic cistern is large. The periotic cistern does not protrude into the fenestra.
Hyobranchial Structures
The first hypobranchial and first ceratobranchial (alternatively homologized as the first ceratobranchial and first epibranchial, respectively) are fused together. The second ceratobranchial (alternatively homologized as the second epibranchial) comprises two elements. Lungs and an ypsiloid cartilage are present except in Onychodactylus. Larvae have four pairs of gill slits.
Characteristics of the Trunk and Vertebral Column
The scapula and coracoid bones of the pectoral girdle are fused to form the scapulocoracoid. Vertebral centra are amphicoelous. Ribs are unicapitate. Neural arches of vertebrae lack foramina, and spinal nerves exit intervertebrally. The pubotibialis and puboischiotibialis muscles are fused together. Anterior glomeruli of the kidney are reduced or absent.
Reproductive Characters
Fertilization is external. Ciliated epithelium is present in the cloacal tube and/or anterior cloacal chamber of females. Epidermal lining is present in the anterior cloacal chamber of females. Evaginations are absent from the dorsolateral walls of the male cloacal tube. Anterior ventral glands are present in the cloacae of females. No spermathecae are present in the female cloacal chamber. Glands secreting into the dorsal walls of the female cloaca are absent. Anterior ventral glands are present in male cloacae. Posterior ventral glands are absent from male cloacae. Kingsbury's glands are absent from male cloacae. Dorsal pelvic glands are absent in males. Lateral pelvic glands are absent in males. Glands secreting into the male cloacal orifice are absent. Parental care of eggs is by males.
The diploid number of chromosomes is 40, 56, 60 or 62 (see Morescalchi, 1975).
Classification
The family Hyobiidae contains approximately 50 species assigned to ten genera (number of species in parentheses): Batrachuperus (6), Hynobius (30), Liua (2), Onychodactylus (2), Pachyhynobius (1), Paradactylodon (3), Protohynobius (1), Pseudohynobius (2), Ranodon (1), and Salamandrella (2) (Frost, 1985; Duellman, 1993; Zhang et al., 2006; AmphibiaWeb: Hynobiidae). Pachypalaminus and Xenobius are no longer recognized (synonymized with Hynobius and Pachyhynobius, respectively).
The family Hynobiidae is fairly closely related to the family Cryptobranchidae (Larson, 1991; Larson and Dimmick, 1993), with which it forms the caudate suborder Cryptobranchoidea (see Duellman and Trueb, 1986). Hynobiids are divided into two subfamilies, the Protohynobiinae (Protohynobius) and the Hynobiinae (all others).
Discussion of Phylogenetic Relationships
The phylogeny presented here is inferred from 15 complete mitochondrial genome sequences (Zhang et al., 2006). Zhang et al. (2006) resurrected the genus Paradactylodon and assigned to it three species from Central Asia (Afghanistan and Iran) formerly assigned to Batrachuperus (B. mustersi, B. persicus, and B. gorganensis). For additional discussion of hynobiid phylogeny, see Larson et al. (2003).
References
Duellman, W. E. 1993. Amphibian Species of the World: Additions and Corrections. Univ. of Kansas Printing Service. Lawrence, KS.
Duellman, W. E. and L. Trueb. 1986. Biology of Amphibians. McGraw-Hill, New York.
Frost, D. R. 1985. Amphibian Species of the World. Allen Press and the Association of Systematics Collections. Lawrence, Kansas.
Hecht, M. K. and J. L. Edwards. 1977. The methodology of phylogenetic inference above the species level. Pp. 3-51 in M. K. Hecht, P. C. Goody and B. M. Hecht (eds.) Major Patterns in Vertebrate Evolution. Plenum Press, New York.
Larson, A. 1991. A molecular perspective on the evolutionary relationships of the salamander families. Evolutionary Biology 25:211-277.
Larson, A. and W. W. Dimmick. 1993. Phylogenetic relationships of the salamander families: A analysis of congruence among morphological and molecular characters. Herpetological Monographs 7:77-93.
Larson, A., D. W. Weisrock, and K. H. Kozak. 2003. Phylogenetic systematics of salamanders (Amphibia: Urodela), a review. Pp. 31-108 in Reproductive Biology and Phylogeny of Urodela (D. M. Sever, ed.) Science Publishers, Inc., Enfield (NH), USA.
Morescalchi, A. 1975. Chromosome evolution in the caudate Amphibia. Evolutionary Biology 8:339-387.
Zhang, P., Y.-Q. Chen, H. Zhou, X.-L. Wang, T. J. Papenfuss, D. B. Wake and L.-H. Qu. 2006. Phylogeny, evolution, and biogeography of Asiatic salamanders (Hynobiidae). Proceedings of the National Academy of Sciences 103:7360-7365.
Zhao, E., Y. Jiang, Q. Hu and Y. Yang. 1988. Studies on Chinese Salamanders. Society for the Study of Amphibians and Reptiles. Oxford, Ohio.
About This Page
Todd Jackman and J. Robert Macey contributed to the preparation of this Tree of Life page.
Allan Larson

Washington University, St. Louis, Missouri, USA
David Wake

University of California, Berkeley, California, USA
Tom Devitt

University of California, Berkeley, California, USA
Correspondence regarding this page should be directed to David Wake at
Page copyright © 2006 Allan Larson, David Wake, and
All Rights Reserved.
- Content changed 05 September 2006
Citing this page:
Larson, Allan, David Wake, and Tom Devitt. 2006. Hynobiidae. Version 05 September 2006 (under construction). http://tolweb.org/Hynobiidae/15453/2006.09.05 in The Tree of Life Web Project, http://tolweb.org/








 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site