Sordariomycetes
Ning Zhang and Gi-Ho Sung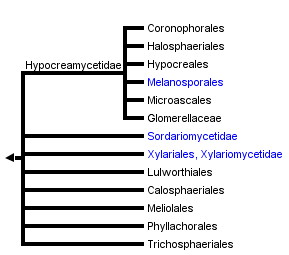

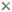
This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
The Sordariomycetes is one of the largest classes in the Ascomycota with more than 600 genera and 3000 known species (Kirk et al 2001). It includes most non-lichenized ascomycetes with perithecial (flask-shaped) or less frequently cleistothecial (non-ostiolate) ascomata and inoperculate unitunicate or prototunicate asci (Alexopolous et al 1996).
The term “pyrenomycetes” was used to unite fungi with perithecial ascomata and unitunicate asci (Luttrell 1951). Its use was discontinued based on the placement of perithecial species outside of the clade and the inclusion of species with prototunicate asci (e.g. Corollospora and Ophiostoma), in order to avoid confusion.
Members of the Sordariomycetes are ubiquitous and cosmopolitan and function in virtually all ecosystems as pathogens and endophytes of plants, arthropod and mammalian pathogens, mycoparasites, and saprobes involved in decomposition and nutrient cycling. The most famous members include Cryphonectria parasitica (the causal agent of chestnut blight), Magnaporthe grisea (the cause of rice blast), and Neurospora crassa (the model organism widely used in molecular and genetic studies).
Characteristics
The majority of Sordariomycetes produce perithecial ascomata (Figs 1,2). The shape, size, pigmentation, texture, and position of ascomata were characters used in traditional taxonomy. For example, the position of ascomata in relation to substrates was used in family delimitation of the Diaporthales by Barr (1978), but this classification was not supported by phylogenetic analyses using molecular characters (Castlebury et al 2002, Zhang and Blackwell 2001).


Figs. 1,2. From left to right, Valsella salicis. Longitudinal section of perithecia. © David Farr; Coniochaeta leucoplaca. Longitudinal section of perithecium. © Sabine M. Huhndorf
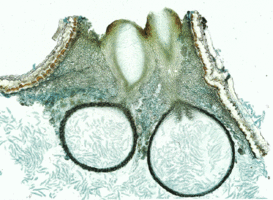
Nannfeldt (1932) and Luttrell (1951) first applied ontogenetic characters in filamentous ascomycete classification. Although the ontogenetic characters do not always correspond well with molecular phylogenies, they are still informative characters in ordinal level classification within the Sordariomycetes (Samuels and Blackwell 2001). The typical arrangement of asci in the Sordariomycetes is basal or peripheral in a hymenium (Figs 1,2). The presence or absence of an apical ring is an important feature in the Sordariomycetes classification (Fig. 3). Typically, asci of the Sordariomycetes are octosporous (Figs 4,5).



Figs. 3-5. From left to right, Jobellisia luteola. Apical ring of ascus. © Andrew Miller; Coniochaeta leucoplaca . Asci with ascospores; Lasiosphaeria ovina. Ascus with ascospores. © Sabine M. Huhndorf.
The Sordariomycetes is an anamorph-rich class, with significant diversity represented by hyphomycete and coelomycete species. Many species of the Hypocreales, Ophiostomatales, and Chaetosphaeriales have two or more distinguishable anamorphs (synanamorphs) (Figs 6-8). Hyphomycetes occur throughout the class, but coelomycete anamorphs also occur, most notably in the Glomerellaceae and Diaporthales. In common with teleomorph characters, many characters used to delimit anamorph genera (e.g. variations in conidiomata, pigmentation, conidiophore branching, and conidial septation) are homoplasic in the Sordariomycetes. Despite this, recognizable patterns of anamorph morphological characters often allow recognition of phylogenetic groups (Seifert and Gams 2001).

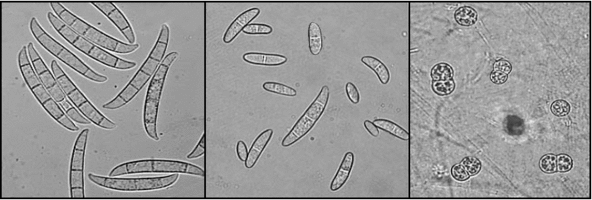
Figs. 6-8. Fusarium solani (teleomorph: Nectria haematococca). From left to right, macroconidia, microconidia, and clamydospores © David Geiser.
Discussion of Phylogenetic Relationships
In the current classification sensu Eriksson (2006), the Sordariomycetes comprises 16 orders in three subclasses (i.e. the Hypocreomycetidae, Sordariomycetidae and Xylariomycetidae). A recent phylogenetic analysis based on multi-gene sequences (Zhang et al. 2006) is largely consistent with the current classification. It supports the monophyly of the Sordariomycetes and the three subclasses. At the ordinal level, however, Microascales is recognized as paraphyletic and Melanosporales is recognized as a distinct order in the Hypocreomycetidae (Zhang et al. 2006).
Phylogenetic studies provide a foundation for developing hypotheses on the dynamic process of evolutionary patterns, and an insight into the long and diverse evolution of the nutritional mode/fungal symbioses in the Sordariomycetes. A synapomorphy of the Sordariomycetes is the perithecial ascoma, which is evolved from the apothecium of ancestral Pezizomycotina (Spatafora et al. 2006). However, taxa in a number of unrelated lineages of the Sordariomycetes have lost ostioles, which is usually associated with the loss of forcible discharge of ascospores (Malloch 1981, Suh and Blackwell 1999). Most members of the Xylariomycetidae and some of the Sordariomycetidae have dark perithecia, amyloid asci, true paraphyses, and periphysate ostioles. These traits may be plesiomorphies in the Sordariomycetes, although the relationships among the three subclasses still are not confidently resolved.
The majority of members of the Sordariomycetes are terrestrial, and life in aquatic habitats is considered a derived character for the class (Samuels and Blackwell 2001). The Halosphaeriales, Sordariales, Diaporthales, Xylariales, and Magnaporthaceae contain freshwater species, while most marine species are classified in the Halosphaeriales and Lulworthiales. Most of these fungi break down lignin and cellulose from plant debris in intertidal and subtidal zones, very rarely also in the deep sea. All the major lineages in the Sordariomycetes contain aquatic species (Shearer 1993, Spatafora et al 1998). The move to aquatic environments may have occurred multiple times in the class. The Xylariomycetidae comprises saprophytes and plant pathogens, which are also abundant in the other two subclasses. Therefore, the saprophytic and plant parasitic habits may be the ancestral states of the Sordariomycetes. Most mycoparasites and insect associates are derived from the Hypocreomycetidae, and the Sordariomycetidae is rich in coprophilous taxa.
References
Alexopoulos CJ, Mims CW, Blackwell M. 1996. Introductory Mycology. New York, USA: John Wiley & Sons, Inc.
Barr ME. 1978. The Diaporthales in North America. Mycol Mem 7:1-232.
Castlebury LA, Rossman AY, Jaklitsch WJ, Vasilyeva LN. 2002. A preliminary overview of the Diaporthales based on large subunit nuclear ribosomal DNA sequences. Mycologia 94:1017-1031.
Eriksson OE, ed. 2006. Outline of Ascomycota - 2006. Myconet. 12:1-82.
Kirk PM, Cannon PF, David JC, Stalpers JA. 2001. Ainsworth & Bisby's Dictionary of the Fungi, 8th ed. Surrey, UK: CABI Bioscience.
Luttrell ES. 1951. Taxonomy of the pyrenomycetes. University of Missouri Studies 24:1-120.
Malloch D. 1981. The plectomycete centrum. In: Reynold DR, editor. Ascomycete systematics. Berlin Heidelberg New York: Springer. p73-91.
Nannfeldt JA. 1932. Studien ?ber die morphologie und systematik der nicht-lichenisierten inoperculaten Discomyceten. Nova Acta Reg Soc Sci Ups IV 8:1-368.
Samuels GJ, Blackwell M. 2001. Pyrenomycetes-fungi with perithecia. In: McLaughlin DJ, McLaughlin EG, Lemke PA, eds. The Mycota VII Part A. Berlin Heidelberg: Springer-Verlag. p221-255.
Seifert KA, Gams W. 2001. The taxonomy of anamorphic fungi. In: McLaughlin DJ, McLaughlin EG, Lemke PA, eds. The Mycota VII Part A. Berlin Heidelberg: Springer-Verlag. p 307-347.
Shearer CJ. 1993. The freshwater ascomycetes. Nova Hedwigia 56:1-33.
Spatafora, JW, Sung, G-H, Johnson, D, Hosaka, K, O?Rourke, B, Serdani, M, Spotts, R, Lutzoni, F, Hofstetter, V, Fraker, E, Gueidan, C, Miadlikowska, J, Reeb, V, Lumbsch, T, L?cking, R, Schmitt, I, Aptroot, A, Roux, C, Miller, A, Geiser, D, Hafellner, J, Hestmark, G, Arnold, AE, B?del, B, Rauhut, A, Hewitt, D, Untereiner, W, Cole, M.S, Scheidegger, C, Schultz, M, Sipman, H. Schoch, C. 2006. A five-gene phylogenetic analysis of the Pezizomycotina. Mycologia 98: 1018-1028.
Spatafora JW, Volkmann-Kohlmeyer B, Kohlmeyer J. 1998. Independent terrestrial origins of the Halosphaeriales (marine Ascomycota). Amer J Bot 85:1569-1580.
Suh SO, Blackwell M. 1999. Molecular phylogeny of the cleistothecial fungi placed in Cephalothecaceae and Pseudeurotiaceae. Mycologia 91:836-848.
Zhang N, Blackwell M. 2001. Molecular phylogeny of dogwood anthracnose fungus (Discula destructiva) and the Diaporthales. Mycologia 93:355-365.
Zhang N, Castlebury, LA, Miller, AN, Hundorf, SM, Schoch, CL, Seifert, KA, Rossman, AY, Rogers, JD, Kohlmeyer, J, Volkmann-Kohlmeyer, B, Sung, G-H. 2006. An overview of the systematics of the Sordariomycetes based on a four-gene phylogeny. Mycologia 98: 1076-1087.
Title Illustrations

From left to right, Hypocrea lutea, perithecia embedded in stromata; Cordyceps scarabaeicola, mature stromata growing on a scarabaeid pupa.
| Scientific Name | Hypocrea lutea |
|---|---|
| Body Part | perithecia embedded in stromata |
| Copyright | © Gary J. Samuels |
About This Page
Ning Zhang

Cornell University, Dept. of Plant Pathology, Ithaca, NY, USA
Gi-Ho Sung

Oregon State University, Corvallis, Oregon, USA
Correspondence regarding this page should be directed to Ning Zhang at and Gi-Ho Sung at
Page copyright © 2007 Ning Zhang and
All Rights Reserved.
- First online 20 November 2007
- Content changed 20 November 2007
Citing this page:
Zhang, Ning and Sung, Gi-Ho. 2007. Sordariomycetes. Version 20 November 2007 (under construction). http://tolweb.org/Sordariomycetes/29050/2007.11.20 in The Tree of Life Web Project, http://tolweb.org/







 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site