Spelerpinae
David Wake and Tom Devitt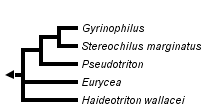


This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxPhylogenetic relationships among members of the subfamily Spelerpinae (taxonomy following Chippindale et al., 2004) after Chippindale et al. (2000, 2004), Hillis et al., (2001), Wiens et al. (2003), and Bonett and Chippindale (2004).
Introduction
Salamanders of the subfamily Spelerpinae are widely distributed across eastern North America, extending as far west as the Interior Highlands (Ozark Plateau and Ouachita Mountains) of the United States and the Edwards Plateau region of central Texas. Populations of Eurycea found in these highland regions likely represent relicts that became restricted to mesic habitats as conditions became more arid (Wake, 1966; Sweet, 1977, 1978, 1982; Chippindale, 2000). Some species exhibit a biphasic life history (Pseudotriton, Eurycea except Paedomolge), while others (Haideotriton, Paedomolge) are obligately aquatic and perennibranchiate, retaining the morphological and ecological characteristics of aquatic larvae throughout life.
Many perennibranchiate species are troglobitic (cave-dwelling), exhibiting a range of morphological features associated with life in caves, such as reduced eyes and skin pigmentation, broadening and flattening of the skull, limb elongation, and reduction in the number of trunk vertebrae (Chippindale, 2000). Multiple instances of convergence (similarity between organisms that is the result of independent adaptation to similar environments, rather than shared evolutionary history) in troglobitic forms have confounded attempts to delimit species and infer phylogenetic relationships based on morphological features (Sweet, 1978, 1984; Chippindale et al., 2000; Wiens et al., 2003). The application of molecular genetic data to spelerpine salamanders has repeatedly uncovered substantial cryptic diversity (Chippindale et al., 2000; Hillis et al., 2001; Bonett and Chippindale, 2004; Kozak et al., 2006).
Characteristics
Detailed Characteristics of the Subfamily Spelerpinae
Characteristics are summarized from Lombard and Wake's (1986) phylogenetic analysis of major plethodontid lineages based on 30 morphological characters with special emphasis on the hyobranchial skeleton and musculature of the feeding system. These characteristics are useful in combination for distinguishing spelerpines from other plethodontids, although the characteristics listed are not synapomorphies of the subfamily Spelerpinae.
Tongue and Hyobranchial Apparatus
Tongue is attached projectile (Haideotriton, Stereochilus, Eurycea (Typhlotriton) spelaeus, Typhlomolge [Eurycea rathbuni, E. robusta, and probably E. waterlooensis]) or free projectile (remaining Eurycea, Gyrinophilus, and Pseudotriton). The hyobranchial skeleton includes a urohyal, an expanded basibranchial, and radii that are discrete, rounded elements tapered towards their tips and distinctly separated from and articulated with the basibranchials. The epibranchial is relatively longer than the basibranchial or first ceratobranchial. The first ceratobranchial is larger than the second ceratobranchial and constitutes the main force-transmitting element in movement of the tongue. The rectus cervicis profundis muscle is linearly arranged. The rectus cervicis superficialis has a lateral slip. The omohydoideus, circumglossus and basiradialis muscles are present. The genioglossus muscle is absent. The intraglossus is attached to the anterior end of basibranchial, lingual cartilage, or equivalent. The anterior section of the hyoglossus muscle is present. The posterior fibers of the hyoglossus are oriented posteriorly. The suprapeduncularis muscle is well developed. The ramus hypoglossus bifurcates distally, near the tip of the basibranchial.
Epibranchial Number
Larvae or embryos have three epibranchials.
Tail Autotomy
Cutaneous wound does not occur, there are 3 caudosacral vertebrae, the first and second caudal vertebrae are normal (not specialized), and tail breakage is not localized.
Brain Stem Motor Column
There are two distinct classes of cells in the motor column of the neck and trunk.
Jaws, Cranial Osteology and Structure of the Inner Ear
The tooth row lies anterior to a bony shelf of the preorbital process. Parietal bones have a distinct ventrolateral shelf. The facial lobe of the maxilla is located in a position near the anterior end of the maxilla, with a distinct section of the pars dentalis extending anterior to it. The premaxillae surround an intermaxillary gland that lies directly behind the pars dentalis, or may be fused, with the intermaxillary gland entirely surrounded and occasionally completely roofed by the bony growth. In the inner ear, the periotic canal forms a ventral loop immediately after leaving the periotic cistern. The bore radius of the otic semiarticular ducts has a negative allometry with respect to body weight.
Chromosome Number
The diploid number of chromosomes is 28.
Development
Some species exhibit a biphasic life history (Pseudotriton, Eurycea except Paedomolge), while others remain perennibranchiate throughout life (Haideotriton, Paedomolge).
Classification
Chippindale et al. (2004) recommended recognizing the subfamily Spelerpinae to represent members of the former tribe Hemidactyliini (Wake, 1966; Lombard and Wake, 1986; Eurycea, Gyrinophilus, Haideotriton, Pseudotriton, and Stereochilus), reserving the name Hemidactylinae for Hemidactylium, based on phylogenetic analysis of molecular and morphological data. Similarly, Macey (2005) recommended placing Hemidactylium in its own subfamily (Hemidactylinae) and recognizing remaining taxa previously assigned to Hemidactyliini in their own subfamily, based on phylogenetic analysis of complete mitochondrial genomic DNA sequences.
Within the Spelerpinae, Mitchell and Reddell (1965) proposed that Typhlomolge be placed in the genus Eurycea, a change subsequently supported by the molecular phylogenetic analysis of Chippindale et al. (2000). Hillis et al. (2001) provided a phylogenetic taxonomy for this group, assigning node-based clade names to several major clades. They proposed retaining the name Typhlomolge to represent the lineage comprising the last common ancestor of E. rathbuni, E. robusta, and E. waterlooensis, plus all of its descendants. Bonnett and Chippindale (2004) reassigned Typhlotriton to the genus Eurycea based on phylogenetic analyses of mitochondrial DNA sequence data, and suggested retaining the name Typhlotriton under a phylogenetic system of classification.
Discussion of Phylogenetic Relationships
Chippindale (2000), Chippindale et al. (1993, 1998, 2000), Hillis et al. (2001) and Wiens et al. (2003) investigated phylogenetic relationships within the central Texas paedomorphic clade of Eurycea (including species formerly assigned to Typhlomolge) using allozymes, mitochondrial DNA sequence data, and morphology. Bonett and Chippindale (2004) used mitochondrial DNA sequence data to investigate phylogenetic relationships among members of the Eurycea multiplicata complex, a clade endemic to the Interior Highlands (Ozark Plateau and Ouachita Mountains) of the United States that includes paedomorphic surface-dwelling, metamorphic surface-dwelling, and metamorphic subterranean members. Kozak et al. (2006) investigated the phylogeography of members of the Eurycea bislineata complex using mitochondrial DNA sequence data, revealing substantial geographically-structured genetic diversity.
References
Bonett, R. M., and P. T. Chippindale. 2004. Speciation, phylogeography and evolution of life history and morphology in plethodontid salamanders of the Eurycea multiplicata complex. Molecular Ecology 13:1189-1203.
Chippindale, P. T. 2000. Species boundaries and species diversity in the central Texas Hemidactyliine plethodontid salamanders, genus Eurycea. Pp. 149-165 in R. C. Bruce, R. G. Jaeger and L. D. Houck, eds. The Biology of Plethodontid Salamanders. Kluwer Academic/Plenum Publishers, New York.
Chippindale, P. T., R. M. Bonett, A. S. Baldwin, and J. J. Wiens. 2004. Phylogenetic evidence for a major reversal of life-history evolution in plethodontid salamanders. Evolution 58:2809-2822.
Chippindale, P. T., A. H. Price, and D. M. Hillis. 1993. A new species of perennibranchiate salamander (Eurycea, Plethodontidae) from Austin, Texas. Herpetologica 49:248-259.
Chippindale, P. T., A. H. Price, and D. M. Hillis. 1998. Systematic status of the San Marcos Salamander, Eurycea nana (Caudata : Plethodontidae). Copeia:1046-1049.
Chippindale, P. T., A. H. Price, J. J. Wiens, and D. M. Hillis. 2000. Phylogenetic relationships and systematic revision of central Texas hemidactyliine plethodontid salamanders. Herpetological Monographs:1-80.
Hillis, D. M., D. A. Chamberlain, T. P. Wilcox, and P. T. Chippindale. 2001. A new species of subterranean blind salamander (Plethodontidae: Hemidactyliini: Eurycea: Typhlomolge) from Austin, Texas, and a systematic revision of central Texas paedomorphic salamanders. Herpetologica 57:266-280.
Kozak, K. H., R. A. Blaine, and A. Larson. 2006. Gene lineages and eastern North American palaeodrainage basins: phylogeography and speciation in salamanders of the Eurycea bislineata species complex. Molecular Ecology 15:191-207.
Lombard, R. E., and D. B. Wake. 1986. Tongue evolution in the lungless salamanders, family Plethodontidae IV. Phylogeny of plethodontid salamanders and the evolution of feeding dynamics. Systematic Zoology 35:532-551.
Mitchell, R. W., and J. R. Reddell. 1965. Eurycea tridentifera a new species of troglobitic salamander from Texas and a reclassification of Typhlomolge rathbuni. Texas Journal of Science 17:343-362.
Sweet, S. S. 1977. Natural metamorphosis in Eurycea neotenes, and generic allocation of Texas Eurycea (Amphibia: Plethodontidae). Herpetologica 33:364-375.
Sweet, S. S. 1978. Status of Eurycea pterophila (Amphibia: Plethodontidae). Herpetologica 34:101-108.
Sweet, S. S. 1982. A distributional analysis of epigean populations of Eurycea neotenes in Central Texas, with comments on the origin of troglobitic populations. Herpetologica 38:430-444.
Sweet, S. S. 1984. Secondary contact and hybridization in the Texas cave salamanders Eurycea neotenes and Eurycea tridentifera. Copeia:428-441.
Wake, D. B. 1966. Comparative osteology and evolution of the lungless salamanders, family Plethodontidae. Memoirs of the Southern California Academy of Sciences 4:1-111.
Wiens, J. J., P. T. Chippindale, and D. M. Hillis. 2003. When are phylogenetic analyses misled by convergence? A case study in Texas cave salamanders. Systematic Biology 52:501-514.
Title Illustrations

| Scientific Name | Pseudotriton ruber nitidus |
|---|---|
| Specimen Condition | Live Specimen |
| Source | Blue Ridge Red Salamander |
| Source Collection | Flickr |
| Image Use |
 This media file is licensed under the Creative Commons Attribution-NonCommercial-ShareAlike License - Version 2.0. This media file is licensed under the Creative Commons Attribution-NonCommercial-ShareAlike License - Version 2.0.
|
| Copyright | © 2004 Jack Goldfarb |
| Scientific Name | Eurycea cirrigera |
|---|---|
| Specimen Condition | Live Specimen |
| Source | Southern 2-lined salamander |
| Source Collection | Flickr |
| Image Use |
 This media file is licensed under the Creative Commons Attribution-NonCommercial-ShareAlike License - Version 2.0. This media file is licensed under the Creative Commons Attribution-NonCommercial-ShareAlike License - Version 2.0.
|
| Copyright | © 2004 Jack Goldfarb |
About This Page
David Wake

University of California, Berkeley, California, USA
Tom Devitt

University of California, Berkeley, California, USA
Correspondence regarding this page should be directed to David Wake at
Page copyright © 2006 David Wake and
All Rights Reserved.
- First online 12 October 2006
- Content changed 12 October 2006
Citing this page:
Wake, David and Tom Devitt. 2006. Spelerpinae. Version 12 October 2006 (under construction). http://tolweb.org/Spelerpinae/66377/2006.10.12 in The Tree of Life Web Project, http://tolweb.org/









 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site