Stylopidia
Jeyaraney KathirithambyClassification after Kinzelbach (1971)
Introduction
The Stylopidia are obligate parasites of other insects. This group is distinguished from the other suborder of Strepsiptera, the Mengenillidia, by the females, which are totally endoparasitic in their hosts. The Stylopidia is the larger of the two suborders, comprising 7 families and 584 species described so far.
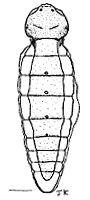
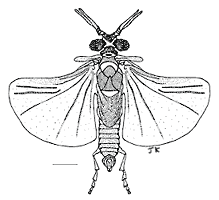
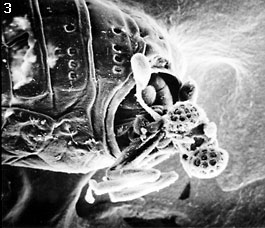
The female Stylopidia possess no external characters typical for normal adult insects, such as eyes, antennae, mouthparts, legs, wings and external genitalia. The only visible feature is the cephalothorax, that extrudes through the host at the neotenic adult stage (fig. 1). The free-living male (fig. 2) emerges from the puparium in the host (fig. 3) and seeks an endoparasitic female and fertilizes it (fig. 4). The extreme sexual dimorphism is most pronounced in this suborder. In the suborder Mengenillidia, both the males and females emerge from the host to pupate externally. For more information on the insects parasitized by stylopidians see the page on Stylopidia Host Relationships.
Fig. 1. Delphacid female with three female Elenchus varleyi Kathirithamby cephalothoraxes (arrows). Image from Kathirithamby (1989b), copyright © 1989 CSIRO Publishing. Fig. 2. Frontal view of male Elenchus tenuicornis (Kirby). Image copyright © 2002 J. Kathirithamby. Fig. 3. Male Elenchus tenuicornis (Kirby) emerging from Javesella dubia (Kirschbaum) (Delphacidae). Image from Kathirithamby (1989a). Copyright © 1989 Blackwell Science. Fig. 4. Male Stylops pacificus Bohart mating with female endoparasitic in Andrena complexa Viereck (Hymenoptera). Photograph by Edward S. Ross, from Kathirithamby (1998a), copyright © 1998 Edward S. Ross.
Most stylopidian families are cosmopolitan in distribution. The only exceptions are:
- Callipharixenidae: now only known from South East Asia (Kinzelbach 1971, Kathirithamby unpublished)
- Bohartillidae: known from the Neotropics (Honduras and Ecuador, Kinzelbach 1969b and Kathirithamby unpublished) and in Dominican amber (Kathirithamby & Grimaldi 1993, Kinzelbach & Pohl 1994)
Characteristics
The autapomorphic characters of the suborder Stylopidia are:
- Neotenic females are totally endoparasitic in the hosts (fig. 1), unlike the Mengenillidia, which emerge during the last larval instar to pupate externally.
- Female Stylopidia have a number of genital openings from the body cavity into the apron.
- MA1 vein in the wing of male with only residues.
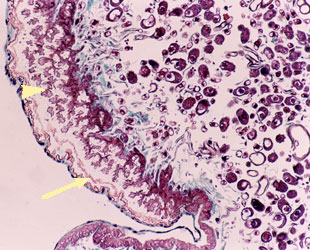
The suborder Stylopidia is distinct from the Mengenillidia, in that the females are totally endoparasitic in their hosts, and remain within their larval cuticles, except for the extruded cephalothorax. They do not undergo a pupal stage but become neotenic adults after the IVth larval stage, when the cephalothorax is extruded. The ventral surface (fig. 5) has a peritrophic matrix (fig. 6) that is analagous to the gut in insects and is referred to as the "brood canal/apron" (Kathirithamby 2000). Several genital tubes open from the body cavity of the female into the apron (fig. 7). The brood canal/apron is initially used for the entry of sperm and later as a passage to the outside for the emergence of the viviparous host-seeking 1st instar larvae from the body cavity of the mother to the outside (fig. 8).
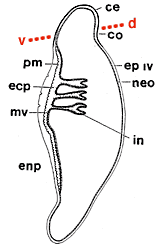
Left: Fig. 5. 5 μm section of integument in the region of the apron in female Stichotrema dallatorreanum Hofeneder showing microvillate cells (arrow head) and peritrophic matrix (arrow). Right: Fig. 6. Neotenic female Stichotrema dallatorreanum Hofeneder. Diagrammatic representation of a longitudinal section revealing the 4th instar unshed cuticle which is sclerotised anteriorly as the cephalothorax (cp) and collar (co), posteriorly as a thin unscleroticed cuticle (ep). The neotenic female (neo) lies within this persistent cuticle. The region of the apron is on the ventral side (v) of the cephalothorax (cp), consisting of a cuticle analogous to the peritrophic matrix (pm). The ectoperitrophic space (ecp) is lined with microvilli (mv), and the anterior abdominal segments have invaginations (in) that lead into the female body cavity. The endoperitrophic space (enp) is the host hemocoel. The dotted line indicates the margin of the host cuticle with dorsal (d) and ventral (v) sides of the neotenic female. Images from Kathirithamby (2000). Copyright © 2000 Blackwell Science.
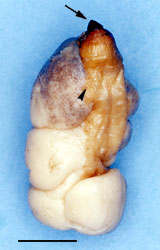
Left: Fig. 7. Neotenic female Stichotrema dallatorreanum Hofeneder with cephalothorax (arrow) and apron (arrow head). Scale bar= 5mm. Image from Kathirithamby et al. (2001). Copyright © 2001 The Netherlands Entomological Society. Right: Fig. 8. SEM of 1st instar larva of Stichotrema dallatorreanum Hofeneder emerging from an invagination/genital tube into the brood canal.
Discussion of Phylogenetic Relationships
There are 7 families in this suborder, but many of the families are described only from females or males such as the females of Bohartillidae and males of Callipharixenidae are unknown, and only six females out of the 106 described Myrmecolacidae are known. Most of the species in the family Myrmecolacidae have been described from free-living males that have come into traps.
While the relationships among stylopidian families are largely unresolved, preliminary evidence indicates a sistergroup relationship between the Myrmecolacidae and Elenchidae. Autapomorphies that distinguish these two groups from the rest of the families are (Kinzelbach 1971):
- Narrow antennal joints
- Short female cephalothorax
- 2-4 genital openings in the female
- Wide brood canal opening
- Mesothoracic tarsus of 1st instar larva pointed
The sister group relationship is also seen in the analysis by Halbert et al. (2001) where seven Myrmecolacidae were compared to Elenchidae and Stylopidae. However, Pohl (2002), who studied the 1st instar larvae, does not support this sister group relationship. A phylogenetic analysis of the molecular data, which is at present being gathered would clarify this question.
References
Bohart, R. M. 1941. A revision of the Strepsiptera with special reference to the species of North America. University of California Publications in Entomology 7(6):91-159.
Brues, C.T. 1903. A contribution to our knowledge of the Stylopidae. Zoologische Jahrbücher Abteilung für Anatomie und Ontogenie der Tiere 18: 241-270.
Halbert, N., L. Ross, J. Kathirithamby, J. B. Wolley, R. Saff and S. Johnston. 2001. Phylogenetic analysis as a means of species Identification within Myrmecolacidae. Tidjschrift voor Entomologie 144: 179-186.
Hubbard, H. G. 1892. The life history of Xenos. Canadian Entomologist 24: 257-261.
Kathirithamby, J. 1978. The effect of stylopization on the sexual development of Javesella dubia (Kirschbaum) (Homoptera). Biological Journal of the Linnean Society 10: 163-179.
Kathirithamby, J. 1979. The effects of stylopization in two species of planthoppers in the Krian District, West Malaysia (Homoptera: Delphacidae). Journal of Zoology London 187: 393-401.
Kathirithamby, J. 1980. Studies of the Nephotettix spp. (Ciciadellidae: Homoptera) in the Krian District, Peninusula Malaysia, Ministry Agriculture Bulletin 51(3): 273-278.
Kathirithamby, J. 1982. Elenchus sp. (Strepsiptera: Elenchidae), a parasite of Nilaparvata lugens (Stal) (Homoptera: Delphacidae) in Peninsular Malaysia. Proceedings of the International Conference of Plant Protection in the Tropics, Kuala Lumpur, pp. 349-361.
Kathirithamby, J. 1989a. Review of the order Strepsiptera. Systematic Entomology 14: 41-92.
Kathirithamby, J. 1989b. Descriptions and biological notes of the Australian Elenchidae (Strepsiptera). Invertebrate Taxonomy 3(2):175-195.
Kathirithamby, J. 1992. Descriptions and biological notes of Halictophagidae (Strepsiptera) from Australia, with a checklist of the world genera and species. Invertebrate Taxonomy 6: 159-196.
Kathirithamby, J. 1998a. Introductory Remarks. International Journal of Insect Morphology and Embryology 27: 1-2.
Kathirithamby, J. 1998b. Host-parasite associations of Strepsiptera: anatomical and developmental consequences. International Journal of Insect Morphology and Embryology 27: 39-51.
Kathirithamby, J. 2000. Morphology of the female Myrmecolacidae (Strepsiptera) including the apron, and an associated structure analogous to the peritrophic matrix. Zoological Journal of the Linnean Society 128: 269-287.
Kathirithamby, J. & D. Grimaldi. 1993. Remarkable stasis in some Lower Tertiary parasitoids: descriptions, new records and review of Strepsiptera in the Oligo-Miocene amber of the Dominican Republic. Entomologica Scandinavica 24(1): 31-41.
Kathirithamby, J. & W. D. Hamilton. 1992. More covert sex: the elusive females of Myrmecolacidae (Strepsiptera). Trends in Ecology and Evolution 7(10): 349-351.
Kathirithamby, J. and S. Johnston. 1992. Stylopisation of Solenopsis invicta (Hymenoptera: Formicidae) by Caenocholax fenyesi (Strepsiptera: Myrmecolacidae). Annals of the Entomological Society of America 85: 293-297.
Kathirithamby, J., S. Simpson, T. Solulu and R. Caudwell. 1998. Strepsiptera parasites – novel biocontrol tools for oil palm integrated pest management in Papua New Guinea. International Journal of Pest Management 44: 127-133.
Kathirithamby, J. and G. Moya-Raygoza. 2000. Halictophagus naulti sp. n. (Strepsiptera: Halictophagidae), a new species parasitic in the corn leafhopper (Homoptera: Cicadellidae). Annals of the Entomological Society of America 93: 1039-1044.
Kathirithamby, J. and K. N. Ponnamma. 2000. Halictophagus palmae sp. nov. (Halictophagus: Strepsiptera) parasitic in Proutista moesta (Westwood) (Homoptera) a vector of phytoplasma diseases of palms in India. Journal of South Asian Natural History 5: 101-105.
Kathirithamby, J., T. Solulu, and R. Caudwell. 2001. Descriptions of female Myrmecolacidae (Strepsiptera) parasitic in Orthoptera (Tettigoniidae) from Papua New Guinea. Tijdschrift voor Entomologie 144: 187-196.
Kinzelbach, R. 1969a. Ein phylogentisches System der Fächerflügler (Insecta: Stepsiptera). Naturwissenschaften 56: 639-640.
Kinzelbach, R. 1969b. Bohartillidae, eine neue Familie der Fächerflügler (Insecta, Strepsiptera). Beitr. neotr. Fauna 6:92-102.
Kinzelbach, R. K. 1971. Morphologische Befunde an Fächerflügern und ihre phylogenetische Bedeutung (Insecta: Strepsiptera). Zoologica 119(1&2) pp. 256.
Kinzelbach, R. K. & H. Pohl, H. 1994. The fossil Strepsiptera (Insecta: Strepsiptera). Annals of the Entomological Society of America 87: 59-70.
Luna de Carvalho, E. 1956. Primeira contribuição para o Estudo dos Estrepsipteros angloenses (Insecta, Strepsiptera). Publicações Culturais da Campanhia de Diamanies de Angola 29: 11-54.
Luna de Carvalho, E. 1959. Segunda contribuição para o Estudo dos Estrepsipteros angloenses (Insecta, Strepsiptera). Publicações Culturais da Campanhia de Diamanies de Angola 41: 125-154;
Luna de Carvalho, E. 1961. Tabela para a determinação dos generos de Estrepsipteros (Insecta). Gracia de Orta 9: 691-698. Garcia de Orta 9: 691-698.
Luna de Carvalho, E. 1967. Terçeira contribuição para o Estudo dos Estrepsipteros angloenses (Insecta, Strepsiptera). Publicações Culturais da Campanhia de Diamanies de Angola 77: 13-66.
Pérez, J. 1886. Des effects du parasitisme des Stylops sur les Apiaires du genre Andrena. Actes de la Société Linnéenne de Bordeaux 4: 21-60.
Pierce, W. D. 1908. A preliminary review of the classification of Strepsiptera. Proceedings of the Entomological Society of Washington 9: 75-85.
Pierce, W. D. 1909. A monographic revision of the twisted winged insects comprising the order Strepsiptera Kirby. Proceedings of the Entomological Society of Washington 66: 1-232.
Pierce, W. D. 1918. The comparative morphology of the Strepsiptera together with records and description of insects. Proceedings of the U.S. National Museum 54: 391-501.
Pohl, H. 2002. Phylogeny of the Strepsiptera based on morphological data of the first instar larvae. Zoologica Scripta 31: 123-138.
Raatikainen, M. 1967. Bionomics, enemies and population dynamics of Javesella pellucida (F.) (Homoptera: Delphacidae). Annales Agriculturae Fennicae 6(2): 3-149.
Riek, E. 1970. Strepsiptera. Insects of Australia, pp. 622-635. University of Melbourne Press.
Salt, G. 1927. The effects of stylopization on Aculeate Hymenoptera. Journal of Experimental Zoology 48: 223-331.
Schrader, S. H. 1924. Reproduction of Acroschismus wheeleri Pierce. Journal of Morphology and Physiology 39: 157-197.
Smith, M. A. G. and A. H. Hamm. 1914. Studies in the experimental analysis of sex. Pt. II. On Stylops and stylopisation. Quarterly Journal of Microscopical Science 60: 435-461.
Ulrich, W. 1927. Über das bisher einzige Strepsipteron aus dem baltischen Bernstein und über eine Theorie der Mengeidenbiologie. Zeitschrift für Morphologie und Ökologie der Tiere 8: 45-62.
Title Illustrations

| Scientific Name | Xenos vesparum Rossi |
|---|---|
| Comments | Neotenic female. Scale bar = 0.8 mm. |
| Sex | Female |
| Copyright |
© 2002 Jeyaraney Kathirithamby

|
| Scientific Name | Xenos vesparum Rossi |
|---|---|
| Comments | Scale bar = 0.8 mm. |
| Sex | Male |
| Copyright |
© 2002 Jeyaraney Kathirithamby

|
| Scientific Name | Xenos vesparum Rossi |
|---|---|
| Specimen Condition | Dead Specimen |
| Sex | Male |
| Body Part | SEM of head |
| Copyright |
© 2002 Jeyaraney Kathirithamby

|
About This Page
Jeyaraney Kathirithamby

University of Oxford, Oxford, UK
Page copyright © 2003 Jeyaraney Kathirithamby
All Rights Reserved.
- First online 20 March 2003
Citing this page:
Kathirithamby, Jeyaraney. 2003. Stylopidia. Version 20 March 2003. http://tolweb.org/Stylopidia/14510/2003.03.20 in The Tree of Life Web Project, http://tolweb.org/













 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site