Published in 1994: Beerli, P., H. Hotz, H. Tunner, S. Heppich, and T. Uzzell 1994. Two new water frog species from the Aegean islands Crete and Karpathos. (Amphibia, Salientia, Ranidae). Notulae Naturae, Academy of Natural Sciences of Philadelphia 470:1-9.
PETER BEERLI, HANSJÜRG HOTZ
Zoologisches Museum, Universität Zürich-Irchel, Winterthurerstrasse
190,
CH-8057 Zürich, Switzerland
HEINZ G. TUNNER, SUSANNA HEPPICH
Biozentrum, Universität Wien, Althanstrasse 14,
A-1090 Wien, Austria
THOMAS UZZELL
Department of Ecology, Ethology and Evolution, University of Illinois,
515 Morrill Hall, 505 South Goodwin Avenue, Urbana, Illinois 61801,
USA
Abstract
The western Palearctic water frogs from Crete are characterized by a unique multilocus combination of electrophoretically detected alleles, including fixed allelic differences at 11 or more of 31 loci, with fixed, unique alleles at 7 loci. The genetic distances to the three nearest mainland taxa in the Balkans and Anatolia are consistent with Crete's 5·106 year geological isolation. Water frogs from Karpathos are also characterized by a unique multilocus combination of electrophoretic alleles, including alleles at 2 of 31 loci that are unique except that they segregate in the population on the neighboring island Rhodos. Phylogenetic analyses of electrophoretic data cluster the Karpathos and Rhodos populations, but geological data suggest that Rhodos was connected to Anatolia well after its separation from Karpathos.
Recent analyses using protein electrophoresis suggest that the water frog populations of the Rana esculenta group occurring on two of the Aegean islands, Crete and Karpathos, are both genetically differentiated from the other western Palearctic water frogs at the species level (Beerli 1993). We here describe these two new species.
Rana cretensis sp. nov.
Holotype
MHNG[12] 2543.90 (field number PB1033), adult male of 63 mm body length from Kastelli, western Crete, Greece (35°30'N/23°43'E), 5 m; collected in October 1987 by P. Beerli.
Paratypes
MHNG 2543.91 (female, field no. 17209), Petros river near Gerani, northern Crete, February 1989, P. Beerli; NMW 33074:1 (female, field no. PB1034), same collecting data as holotype; NMW 33074:2 (female, field number PB1037), Lavris, valley of the river Geropotamos, northern Crete, October 1987, P. Beerli; ANSP[12] 35280 (female, field no. 17210), Petros river; ANSP 35281 (female, field no. PB1035), same collecting data as holotype.
Specimens examined
We examined a total of 41 individuals (including the type series): Type locality, 13 (3 females, 1 male, 9 juveniles); Petros river, 2 (females); Lavris, 6 (4 females, 2 males); Iraklion (river in town), 7 (juveniles); Elaphonissi (extreme southwest corner of Crete, near sea shore, collected in April 1982 by H. Maier), 13 (juveniles). Frozen tissue samples of the type series and other specimens examined are stored at the Zoologisches Museum, Universität Zürich.
Diagnosis and Description
A member of the western Palearctic water frog group, distinguished from the Palearctic brown frog group by paired external lateral vocal sacs in males, extension of webbing of feet to the toe tips, absence of a black face mask from the eye to the tympanum, and presence of dark mottling on the inner thigh surfaces; distinguished from the eastern Palearctic water frog group by protein electrophoretic data: among the loci sharing no alleles with eastern Palearctic water frogs (at least 50%; cf. Nishioka and Sumida 1992), those at aGDH, LDH-B, sMDH[=MDH-1], MPI, and 6PGDH are shared with one to many species of western Palearctic water frogs, whereas those at LDH-A, mMDH[=MDH-2], and sSOD[=SOD-1] are shared with all. Rana cretensis is discriminated from all other western Palearctic water frog species by a unique multilocus combination of electrophoretically detected alleles: among alleles shared with other species, our samples are fixed for mACO c, AHH a, CK-A b, aGDH b, mIDH[=IDH-2] b; LDH-B d, sMDH a, MPI q; MPR1[=MProt1] b, 6PGDH e, PGM-2 d, sSOD a. Rana cretensis has unique ([[ordfeminine]]private[[ordmasculine]]) alleles, all fixed in our samples, at the loci sAAT[=AAT-1] (m), sACO (d), ALB (k), EST-5 (f), EST-6 (d), GPI (h), sIDH[=IDH-1] (g). Centromeres of the 2N=26 chromosomes are conspicuously fluorescent after Actinomycin D / 33258 Hoechst double staining. Morphologically (Fig. 1), among western Palearctic water frogs Rana cretensis is medium-sized (mean ± standard deviation of body length 64.6±9.5 mm for 8 adults). Relative tibia length medium (body length/tibia length 1.97±0.15, N=37). Callus internus relatively short (body length/callus internus length 18.8±2.7, N=31), of medium height (callus length/height 3.4±0.6, N=8), slightly rounded. Digitus primus medium-sized (body length/digitus primus length 7.3±0.8, N=37; digitus primus length/callus internus length 2.4±0.3, N=37). Dorsal coloration light gray to brown with brown to olive-gray spots, occasionally grass-green with distinct brown spots. Venter and throat uniformly whitish with slight gray pigmentation. No vertebral stripe in our samples. Marked yellow pigmentation on posterior flank and inner side of femur and tibia. Prominent broad brown dorsolateral fold. Vocal sacs of males dark gray. The advertisement calls of males (Fig. 2A,C; subsequent values are mean±SD and refer to water temperature 20°C, air temperature 26°C) are produced in long series, with call durations of 646±58 msec and intercall intervals of 612±30 msec. The calls consist of a large number (12.3±1.5) of relatively short (32±4 msec) pulse groups with short (19±5 msec) intervals. These pulse groups contain a further level of amplitude subdivision into 3-7 (5.0±1.2) distinct short (6.4±1.8 msec) pulse groups with virtually no intervals, each with an amplitude maximum near the beginning and then a gradual decline, that consist of 12.4±3.6 pulses.
Derivatio nominis
The name cretensis refers to the island Crete (Kriti, Greece), where this species is the only water frog taxon presently known to occur.
Distribution. Known only from Crete, where it is widely distributed; our samples are all from below 100 m above sea level.


Fig. 1. Rana cretensis: adult female (paratype, MHNG 2543.91) of 72 mm body length from Petros river, Crete.

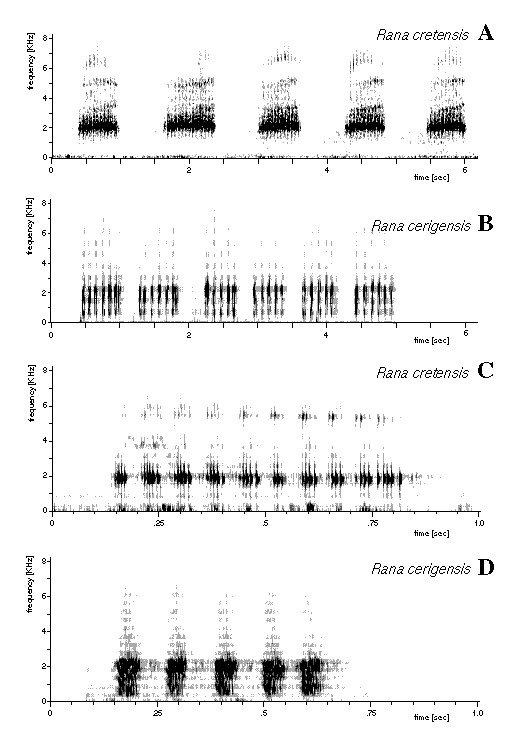
Fig. 2. Sonograms of the advertisement call of an individual Rana cretensis from Petros river, Crete, water temperature 20°C, air temperature 26°C (A,C), and of an individual Rana cerigensis from Olympos, Karpathos, water temperature 20°C, air temperature 18°C (B,D).
Rana cerigensis sp. nov.
Holotype
MHNG[12] 2543.92 (field number 17420), adult male of 41 mm body length from Olympos [[[Omicron]][[lambda]][[upsilon]]u[[pi]][[omicron]][[varsigma]]], northern Karpathos, Greece (35°44'N/27°10'E), ~300 m; collected in April 1990 by P. Beerli.
Paratypes
MHNG 2543.93 (female, field no. 17208), same collecting data as holotype; NMW[12] 33075:1 (female, field no. 17221), same collecting data as holotype; ANSP[12] 35282 (female, field no. 17207), same collecting data as holotype.
Specimens examined
We examined a total of 9 individuals (4 females, 1 male, 4 juveniles) from the type locality. Frozen tissue samples of the type series and other specimens examined are stored at the Zoologisches Museum, Universität Zürich.
Diagnosis and Description
A member of the western Palearctic water frog group, distinguished from the Palearctic brown frog group by paired external lateral vocal sacs in males, extension of webbing of feet to the toe tips, absence of a black face mask from the eye to the tympanum, and presence of dark mottling on the inner thigh surfaces; distinguished from the eastern Palearctic water frog group by protein electrophoresis: among the loci sharing no alleles with eastern Palearctic water frogs, those at [[alpha]]GDH, LDH-B, sMDH, MPI, and 6PGDH are shared with one to several species of western Palearctic water frogs, whereas those at LDH-A, mMDH, and sSOD are shared with all. Rana cerigensis is discriminated from all other western Palearctic water frog species by a unique multilocus combination of electrophoretically detected alleles: among alleles shared with other species, our samples are fixed for mACO b, sACO b, ALB b, CK-A b, EST-6 b, aGDH b, GPI d, sIDH b, mIDH c, LDH-B a, sMDH b, MPI q; 6PGDH e, PGM-2 d, sSOD a. Except that they segregate in the water frog population of Rhodos, Rana cerigensis has unique alleles, fixed in our sample, for sAAT (l) and AHH (c). Morphologically (Fig. 3), among western Palearctic water frogs Rana cerigensis is medium-sized (mean ± SD of body length 54.5±12.2 mm for 8 adults). Tibia relatively long (body length/tibia length 1.85±0.08, N=8). Callus internus relatively short (body length/callus internus length 18.5±2.2, N=7), of medium height (callus length/height 3.2±0.3, N=3). Digitus primus medium-sized (body length/digitus primus length 7.4±1.1, N=7; digitus primus length/callus internus length 2.5±0.3, N=7). Dorsal coloration light brownish gray to olive, with or without light brown spots. No vertebral stripe in our samples. Venter cream-colored with gray mottling. Vocal sacs of males dark gray. The advertisement calls of males (Fig. 2B,D; subsequent values are mean±SD and refer to water temperature 20ëC, air temperature 18ëC) are produced in series, with call durations of 341±44 msec and very short intercall intervals (276±54 msec). The calls consist of a relatively low number (6.0±0.9) of pulse groups, with durations of 57±5 msec and group intervals of 41±15 msec, that comprise a large number (>100) of pulses. In contrast to Rana cretensis, no further level of amplitude subdivision of the pulse groups was observed.
Derivatio nominis
The name cerigensis derives from Cerigo, the Latin name for the island Karpathos, Greece, where this species is the only water frog taxon.
Distribution. Karpathos, possibly Rhodos.


Fig. 3. Rana cerigensis: adult female (paratype, MHNG 2543.93) of 76 mm body length from Olympos, Karpathos.
Discussion
Several islands in the Aegean Sea have been isolated for a long time, up to five million years. Some of these are inhabited by water frogs of the western Palearctic Rana esculenta group. For these frogs, as for other amphibians, the salt water surrounding the islands is an effective dispersal barrier, because the skin of these fresh-water animals is readily permeable to both salt and water. Barring introduction by humans, the geologically determined isolation time of such islands is, therefore, the minimal isolation time between the respective frog populations and thus their minimal genetic divergence time.
The island Crete, although isolated during most of the Miocene, became connected to the surrounding mainlands when the Mediterranean basin dried up 6 million years ago (salinity crisis); it subsequently became completely isolated again 5 million years ago (at the end of the Messinian period) by filling of the dry Mediterranean basin following the re-opening of the Strait of Gibraltar (Hsü et al. 1977). Analysis of the complex tectonic movements in the Aegean region and fossil data suggest that the island Karpathos, between Crete and Rhodos, was connected to Rhodos and the Anatolian mainland in the early Pliocene, and became completely isolated in middle Pliocene (Meulenkamp 1985).
This scenario indicates that the water frogs living on Crete diverged from the other western Palearctic water frogs a minimum of 5 million years and a maximum of 6 million years ago, and that the water frogs of Karpathos diverged from Anatolian relatives about 3 million years ago. Genetic data obtained by protein electrophoresis are consistent with these datings (Beerli 1993).
The population on Crete has similar genetic distances to the three geographically nearest mainland water frog taxa, Rana ridibunda and Rana epeirotica from the Balkan peninsula, and Rana bedriagae 3[4] from Anatolia (Table 1). These distances are among the lowest found between Rana cretensis and any other western Palearctic water frog taxon (Table 1); but they are similar to or higher than genetic distances between European taxon pairs that are known by sympatry not to be conspecific (Rana ridibunda-Rana lessonae, Rana ridibunda-Rana shqiperica, Rana ridibunda-Rana epeirotica; Beerli 1993, Hotz, Beerli and Uzzell, unpublished data). This and the large number of fixed allelic differences (minimum in pairwise comparisons: 11 of 31 loci; 7 of 31 loci fixed for unique alleles) clearly indicate that Rana cretensis is differentiated from the other western Palearctic water frogs at the species level.
Table 1. Genetic distances of Rana cretensis and Rana cerigensis from the presently known western Palearctic water frog taxa (protein electrophoresis, 31 loci; data from Beerli 1993). Values are means of Nei's (1972) standard genetic distance as modified by Hillis (1984), and of Cavalli-Sforza and Edwards' (1967) chord distance.
| Rana cretensis | Rana cerigensis | |||
|---|---|---|---|---|
| Nei/Hillis | Cavalli-Sforza and Edwards | Nei/Hillis | Cavalli-Sforza and Edwards | |
| Rana cretensis | - | - | 0.54 | 0.58 |
| Rhodos population | 0.55 | 0.59 | 0.12 | 0.29 |
| Rana bedriagae Anatolia |
0.58 | 0.58 | 0.32 | 0.44 |
| Rana ridibunda Balkan |
0.65 | 0.62 | 0.27 | 0.44 |
| Rana ridibunda Central Europe |
0.70 | 0.64 | 0.30 | 0.46 |
| Rana epeirotica | 0.53 | 0.57 | 0.49 | 0.56 |
| Rana shqiperica | 0.66 | 0.63 | 0.49 | 0.56 |
| Rana lessonae Central Europe |
0.89 | 0.69 | 0.51 | 0.57 |
| Rana perezi | 0.80 | 0.67 | 0.73 | 0.65 |
| Rana saharica Morocco |
1.35 | 0.77 | 0.95 | 0.71 |
By genetic distance measures (Table 1), Rana cretensis is closest to the western Greek Rana epeirotica. Nevertheless, the differences in genetic distances between Rana cretensis and several other species, and in numbers of fixed allelic differences distinguishing Rana cretensis from these species (including the geographically nearest mainland taxa, Rana ridibunda in Greece and Rana bedriagae in Anatolia) are too small to determine its closest relative reliably. A very conservative cladistically-derived tree of relationships as well as a robust maximum likelihood tree both place Rana cretensis, Rana epeirotica, Rana shqiperica, Rana lessonae, and the Rana ridibunda stock (Rana cerigensis, Rana bedriagae, Rana ridibunda) together in an unresolved group (Beerli 1993). The conspicuous centromeric fluorescence shown by Rana cretensis chromosomes following Actinomycin D/33258 Hoechst double staining resembles that shown by double-stained chromosomes of the Rana ridibunda stock (cf. Heppich et al. 1982; Bucci et al. 1990; Tunner and Heppich-Tunner 1991); whether this reflects convergence or common inheritance remains to be tested. In our phylogenetic analyses, Rana perezi and Rana saharica consistently form an outgroup pair.
Compared to Rana cretensis, Rana cerigensis is less markedly different from Rana bedriagae, the genetically and geographically closest mainland taxon (Table 1; fixed allelic differences at 2 of 31 loci). Speciation within the Rana ridibunda stock needs further study through both molecular genetics and crossing experiments. In all our cladistic and phenetic analyses of protein electrophoretic data, however, Rana cerigensis, while clearly belonging to the Rana ridibunda stock, is the most divergent member of it, and is clustered with the animals from the neighboring island Rhodos as a distinctive unit. If Rana bedriagae is recognized as a species distinct from Rana ridibunda (cf. Schneider et al. 1992), which is consistent with although not required by our present genetic data (Beerli 1993; unpublished results), then Rana cerigensis, which is genetically more distinct, should also be recognized.
Although our analyses of the electrophoretic data cluster the frogs from Karpathos and Rhodos, which suggests including the Rhodos population within Rana cerigensis, the geological history clusters Rhodos with the Anatolian mainland: it became separated from Karpathos ~3 million years ago and from the Anatolian mainland in early Pleistocene, ~1.8 million years ago (Meulenkamp 1985). If these datings are correct, the Karpathos and Rhodos populations do not share an independent evolutionary history. The phylogenetic clustering results from alleles present in the Rhodos population (at 30 of 31 loci) that are shared (exclusively so for sAAT l, AHH c, and EST-5 e) with the Karpathos population; at five of these loci (sAAT, AHH, CA-2, mIDH, MPI), however, the Rhodos population segregates for alternative alleles that occur in Rana bedriagae on the adjacent Anatolian mainland. If the geological history is correct, these data may reflect alleles of ancestral populations that subsequently became extinct in Anatolia. Additional molecular data are needed to determine the evolutionary history and taxonomic status of the water frogs on Rhodos.
There are few water frog populations on the dry island of Karpathos, and population sizes appear to be modest. Given deforestation, scarcity of surface water, and degradation of aquatic habitat by human activity, the long-term survival of Rana cerigensis on Karpathos is uncertain: there is serious danger of its becoming extinct.
Our view of the phyletic radiation of the western Palearctic water frog group has changed substantially in the past two decades. Two species were recognized by Mertens and Wermuth (1960), but evolutionary studies evoked by the hemiclonal reproduction that characterizes widespread natural hybrid lineages (reviewed by Graf and Polls Pelaz 1989, Gönther 1990) have resulted in recognition of at least 9 species today. Most of our current ideas on phylogenetic relationships among western Palearctic water frogs have been shaped by genetic data using biochemical and molecular markers. The two new species described here, like the discovery of Rana shqiperica and of Rana epeirotica (Hotz and Uzzell 1982, Tunner and Heppich 1982), and the recognition of Rana perezi and of Rana saharica as distinct species (Graf et al. 1977, Uzzell 1982; unpublished data), reflect the importance of molecular data in alpha taxonomy.
Footnotes
MHNG = Musée histoire naturelle de GenèveANSP - American natural history survey Philadelphia
NMW -
 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site