Monocotyledons
William J. Hahn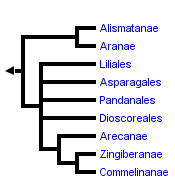


This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
The Monocotyledons are one of the most distinctive major lineages of angiosperms and traditionally have been paired with the Dicotyledons as the two main groups of flowering plants. Among the monocots are some of the largest families of angiosperms (such as the orchids with ca. 20,000 species and the grasses with ca. 15,000 species) as well as some of the most economically important species of plants. Numerous vegetation types are dominated by monocots including grasslands, palm savannas, sedge meadows, and cattail marshes.
Characteristics
The shared, derived character that unites all monocots is the single cotyledon, a feature first noted by John Ray in 1703. Other features that are common in monocots but not necessarily unique to or universal among them are: vascular bundles irregularly distributed in cross section of the stem, parallel veins in the leaves (mostly in the more derived groups), and flower parts in multiples of three. Despite the lack of true secondary growth in monocots, most growth habits are found in the group including floating and submerged aquatics, lianas, trees, epiphytes, and forbs of all sizes.
Relationships of Monocots to other Angiosperms
A singular origin for the monocots is generally accepted with numerous morphological synapomorphies proposed for the group (Donoghue and Doyle, 1989; Doyle and Donoghue, 1992; Tucker and Douglas, 1996). Additionally, most molecular analyses of monocots have provided strong support for their monophyly (Chase, et al., 1993, 1995; Duvall, et al., 1993; Hamby and Zimmer, 1992).
Relative to other angiosperms, the monocots are most frequently placed among a group of predominantly herbaceous magnoliid taxa (Lactoridaceae, Aristolochiaceae, Piperaceae, Sauraraceae, Nymphaeaceae) collectively referred to as the "paleoherbs" (Donoghue and Doyle, 1989). The specific relationships among these taxa are still unclear but a close relationship of the monocots with either the woody magnoliid taxa or the more derived, tricolpate, "eudicots" is not supported in any analysis.
The fossil record of the Monocotyledons is old with the first probable monocot remains dating to the Early Cretaceous. The presence of morphologically diverse fossils by the Campanian suggests a relatively rapid radiation into most of the extant major groups early in the course of monocot evolution (Herendeen and Crane, 1995).
Discussion of Phylogenetic Relationships
The terminal taxa in this phylogeny are based on the major groups recognized and delimited by Dahlgren, et al. (1985) and Thorne (1992a, b) with several modifications. Although earlier work by Huber (1969, 1977) provided the context for a more explicit analysis of monocot relationships, Dahlgren and co-workers were the first to utilize cladistic approaches to the study of monocot phylogeny and their taxonomy is still the most complete. As such, it has served as the basis for most recent studies of the monocots (e.g., Rudall, et al, 1995). Alternate taxonomic systems of Cronquist (1981), Thorne (1992a, b) and Takhtajan (1980, 1987) are largely congruent with that of Dahlgren, et al. (1985) but differ in treatment of lilioid and basal monocots. The systems of Dahlgren and Thorne maintain numerous smaller families in the Lilianae as opposed to a few relatively large ones and place lilioid elements at the base of the monocots rather than the Alismatanae and Aranae.
The phylogenetic hypothesis represented here is based largely on the rbcL reconstructions of Duvall, et al. (1993a, b) and Chase, et al. (1995a) with consideration of the morphological studies of Dalhgren, et al. (1983, 1985) and Stevenson and Loconte (1995; see also Chase, et al., 1995b) as well as the nrDNA 18S SSU studies of Hamby and Zimmer (1992) and Bharathan and Zimmer (1995). Resolution within and among certain subgroups is predominantly based on various works presented in Rudall, et al. (1995).
The general relationships depicted in this tree are recovered in most analyses although taxon sampling is not completely comparable from study to study. Differences in topology concern the nature of the basal lineages (specifically the position of the alismatid/aroid and the dioscorealean/melanthioid lineages), placement of certain enigmatic taxa such as the Cyclanthaceae, Eriocaulaceae, Hanguanaceae, Pandanaceae, Velloziaceae, and Xyridaceae, and resolution within the Lilianae and Commelinanae.
While some earlier studies had suggested basal positions for groups such as the palms (Arecanae), most recent analyses place either the Alismatanae and Aranae (e.g., Duvall, et al., 1993a, 1993b; Chase, et al., 1993, 1995), or members of the lilioid orders Dioscoreales (e.g., Huber, 1969; Dahlgren et al. 1983, 1985; Stevenson and Loconte, 1995) and Melanthiales (e.g., Thorne, 1992; Takhtajan, 1969) as sister to the remainder of the monocots. Much of the difference seen in these two alternative hypotheses is due to uncertainties over the position of monocots within the angiosperms and attendant ambiguity in outgroup choice. Character state homologies are not obvious in many cases and alternate outgroups provide different character state polarizations. The current weight of evidence, however, seems to support the alismatid/aroid hypothesis. An example of this is the trans-spliced structure of the 4th intron in the mitochondrial gene nad1 shared by all monocots sampled except Araceae and Alismataceae (Qui & Palmer, 1996).
References
Bharathan, G & EA Zimmer. 1995. pp 81-107 in: PJ Rudall, PJ Crib, DF Cutler & CJ Humphries. (eds.) Monocotyledons: systematics and evolution,. Royal Botanic Garden, Kew.
Chase, M. W. 2004. Monocot relationships: an overview. American Journal of Botany 91: 1645-1655.
Chase, MW, MR Duval, HG Hills, JG Conran, AV Cox, LE Eguiarte, J Hartwell, MF Fay, LR Caddick, KM Cameron, & S Hoot. 1995a. Molecular phylogenetics of Lilianae. pp 109-137 in: PJ Rudall, PJ Crib, DF Cutler & CJ Humphries (eds.). Monocotyledons: systematics and evolution. Royal Botanic Garden, Kew.
Chase, MW, DW Stevenson, P Wilkin, & PJ Rudall. 1995b. Monocot systematics: a combined analysis. pp 685-730 in: PJ Rudall, PJ Cribb, DF Cutler & CJ Humphries (eds.). Monocotyledons: systematics and evolution. Royal Botanic Garden, Kew.
Cronquist, A 1981. An Integrated System of Classification of the Flowering Plants. 1262 pp. Columbia Univ. Press, New York.
Dahlgren, RMT & HT Clifford. 1982. The Monocotyledons - A Comparative Study. Academic Press, New York.
Dahlgren, RMT, HT Clifford, & PF Yeo. 1985. The Families of the Monocotyledons. 520 pp. Springer-Verlag, Berlin.
Dahlgren, RMT, & F Rassmussen. 1983. Monocotyledon evolution. Characters and phylolgenetic estimation. Evol. Biol. 16: 255-395.
Donoghue, MJ & JA Doyle. 1989. Phylogenetic analysis of angiosperms and the relationships of Hamemelidae. pp 17-45 in: PR Crane & S Blackmore (eds.). Evolution, Systematics, and the Fossil History of the Hamamelidae vol. 1. Clarendon Press, Oxford.
Doyle, JA & MJ Donoghue. 1992. Fossils and seed plant phylogeny realalyzed. Brittonia 44: 89-106.
Doyle, JA, MJ Donoghue, & EA Zimmer. 1994. Integration of morphological and rRNA data on the origin of angiosperms. Ann. Missouri Bot. Gard. 81: 419-450.
Duvall, MR, GH Learn, LE Eguiarte, & ME Clegg. 1993a. Phylogenetic analysis of rbcL sequences identifies Acorus calamus as the primal extant monocotyledon. Proc. Natl. Acad. Sci. USA 90: 4641-4644.
Duvall, MW, MT Clegg, MW Chase, WD Clark, WJ Kress, HG Hills, LE Eguiarte, JF Smith, BS Gaut, EA Zimmer, GH Learn, Jr. 1993b. Phylogenetic hypotheses for the monocotyledons constructed from rbcL sequence data. Ann. Missouri Bot. Gard. 80: 607-619.
Grayum, M 1990. Evolution and phylogeny of the Araceae. Ann. Missouri Bot. Gard. 77: 628-697.
Hamby, RK & EA Zimmer. 1992. Ribosomal RNA as a phylogenetic tool in plant systematics. pp. 50-91 in: PS Soltis, DE Soltis, & JJ Doyle (eds) Molecular Systematics of Plants. Chapman and Hall, New York.
Herendeen, PS, & PR Crane. 1995. The fossil history of the monocotyledons. pp. 1-21 in: PJ Rudall, PJ Cribb, DF Cutler & CJ Humphries (eds.). Monocotyledons: systematics and evolution. Royal Botanic Garden, Kew.
Huber, H 1969. Die Samenmerkmale und Verwandtschaftsverhaltnisse der Liliiflorae. Mitt. Bot. Staatssamml. Munchen 8: 219-538.
Huber, H 1977. The treatment of the monocotyledons in an evolutionary system of classification. Pl. Syst. Evol., Suppl. 1: 285-298.
Les, DD & RR Haynes. 1995. Systematics of subclass Alismatidae: A synthesis of approaches. pp 353-377 in: PJ Rudall, PJ Cribb, DF Cutler & CJ Humphries (eds.). Monocotyledons: systematics and evolution. Royal Botanic Garden, Kew.
Linder, HP & EA Kellogg. 1995. Phylogenetic patterns in the commelinid clade. pp 473-496 in: PJ Rudall, PJ Cribb, DF Cutler & CJ Humphries (eds.). Monocotyledons: systematics and evolution. Royal Botanic Garden, Kew.
Loconte, H & DW Stevenson. 1991. Cladistics of the Magnoliidae. Cladistics 7: 267-296.
Qiu, Y-L & JD Palmer. 1996. Intron evolutionand angiosperm phylogeny. Amer. J. Bot. 83(6-supplement): 188.
Rudall, PJ, PJ Cribb, DF Cutler & CJ Humphries. (eds.) 1995. Monocotyledons: systematics and evolution. Royal Botanic Garden, Kew.
Stevenson, DW & H Loconte, 1995. Cladistic analysis of monocot families. pp 543-578 in: PJ Rudall, PJ Cribb, DF Cutler & CJ Humphries (eds.). Monocotyledons: systematics and evolution. Royal Botanic Garden, Kew.
Takhtahan, A. 1980. Outline of the classification of flowering plants (Magnoliophyta). Bot. Rev. 46: 225-359.
Takhtajan, A 1987. Systema Magnoliophytorum. Nauka, Leningrad. [In Russian].
Thorne, RF 1992a. Classification and geography of the flowering plants. Bot. Rev. 58: 225-348.
Thorne, RF 1992b. An updated classification of the flowering plants. Aliso 13: 365-389.
Tucker, SC & AW Douglas. 1996. Floral structure, development and relationships of Paleoherbs: Saruma, Cabomba, Lactoris, and selected Piperales. pp. 141-175 in: DW Taylor & LJ Hickey (eds.), Flowering Plant Origin, Evolution, & Phylogeny. Chapman & Hall. New York.
Title Illustrations

| Scientific Name | Amorphophallus |
|---|---|
| Image Use |
 This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0. This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0.
|
| Copyright |
© 1997

|
| Scientific Name | Hosta plantaginea (Lam.) Asch. |
|---|---|
| Image Use |
 This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0. This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0.
|
| Copyright |
© 1997

|
| Scientific Name | Pandanus |
|---|---|
| Image Use |
 This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0. This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0.
|
| Copyright |
© 1997

|
| Scientific Name | Butomus umbellatus |
|---|---|
| Location | Ithaca, New York, USA |
| Specimen Condition | Live Specimen |
| Body Part | flowers |
| Source | Prettiest rush ever... |
| Source Collection | Flickr |
| Image Use |
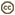 This media file is licensed under the Creative Commons Attribution-NonCommercial-ShareAlike License - Version 2.0. This media file is licensed under the Creative Commons Attribution-NonCommercial-ShareAlike License - Version 2.0.
|
| Copyright | © 2007 Jenn Forman Orth |
About This Page

Georgetown University, Washington, D. C., USA
Correspondence regarding this page should be directed to William J. Hahn at
Page copyright © 1997
 Page: Tree of Life
Monocotyledons.
Authored by
William J. Hahn.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Monocotyledons.
Authored by
William J. Hahn.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Citing this page:
Hahn, William J. 1997. Monocotyledons. Version 01 January 1997 (under construction). http://tolweb.org/Monocotyledons/20668/1997.01.01 in The Tree of Life Web Project, http://tolweb.org/







 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site