Agaricomycetes
Mushroom-Forming Fungi
David S. Hibbett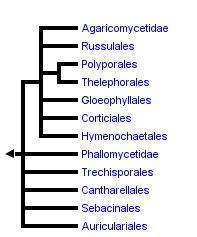


This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
The Agaricomycetes contains approximately 16,000 described species, which is 98% of the described species in the Agaricomycotina (Kirk et al. 2001). Agaricomycetes produce mushrooms, and are therefore the most familiar and conspicuous of all Fungi. Other Fungi produce macroscopic fruiting bodies as well, but the diversity of forms in the Agaricomycetes is unmatched.
Fruiting bodies of Agaricomycetes range from millimeter-scale cyphelloid forms, which look like tiny cups, to the giant polypores Rigidoporus ulmarius (up to 316 kg; see the Fungi page) and Bridgeoporus nobilissimus (up to 130 kg; Burdsall et al. 1996). Agaricomycetes include not only the largest fruiting bodies in Fungi, but perhaps the largest and oldest individuals in any group of organisms. Clones of the honey mushroom, Armillaria gallica, produce average-sized mushrooms, but their mycelial networks have been estimated to cover areas up to 15 hectares, with a mass of 10,000 kg (comparable to a blue whale), and an age of 1500 years (Smith et al. 1992).
Agaricomycetes function as decayers, pathogens, parasites, and mutualistic symbionts of both plants and animals. They make their broadest ecological impacts through their activities as wood-decayers and ectomycorrhizal symbionts of forest trees (such as pines, oaks, dipterocarps, and eucalypts; Rayner and Boddy 1988; Smith and Read 1997). Agaricomycetes are widespread in virtually all terrestrial ecosystems, and a few have secondarily returned to aquatic habitats.
The majority of edible mushrooms are Agaricomycetes (truffles and morels are in the Ascomycota, however). Cultivated edible Agaricomycetes are decayers that have been domesticated, such as button mushrooms (Agaricus bisporus), shiitake (Lentinula edodes), oyster mushrooms (Pleurotus ostreatus), and others. Most of the wild-collected edible species are mycorrhizal (making them difficult or impossible to cultivate), such as porcini (Boletus edulis), chanterelles (Cantharellus cibarius), and matsutake (Tricholoma matsutake). Would-be mushroom hunters should be aware that some species of Agaricomycetes produce secondary metabolites that render them toxic or hallucinogenic (or bioluminescent; Fig. 1).
Mushrooms have been used for medicinal and spiritual purposes in diverse human societies. For example, the 5300-year-old Ice Man who was discovered in a Tyrolean glacier was found carrying pieces of the birch polypore, Piptoporus betulinus, which he may have been using to treat intestinal parasites (Capasso 1998). Another fascinating item of ethnomycology comes from the Pacific Northwest of the United States, where Indigenous People have carved figurines out of the fruiting bodies of the polypore Fomitopsis officinalis to serve as guardians at the graves of shamans (Fig. 1; Blanchette et al. 1992).
Figure 1. Magic mushrooms. Left: The bioluminescent Agaricomycete, Panellus stypticus (Agaricales), photographed by its own light. © D. Hibbett. Right: Grave guardian carved from a fruiting body of Fomitopsis officinalis (Polyporales). © Mycological Society of America. From Blanchette et al. (1992); used with permission.
Characteristics
The generalized life cycle described and illustrated on the Basidiomycota page applies to Agaricomycetes. These fungi are almost always filamentous, without yeast phases. However, yeasts are produced by some Agaricomycetes that are cultivated in underground fungus gardens by attine "leaf-cutter" ants in the neotropics (Mueller et al. 1998). In filamentous forms the hyphae are divided by septa, which are penetrated by a barrel-shaped dolipore that is flanked by membrane-bound parenthesomes (see the Agaricomycotina page for an illustration of septal structure). The dominant stage in the life cycle is typically a dikaryotic mycelium (binucleate, with two haploid nuclei derived from mating), but stable diploids have been reported in Armillaria (Anderson 1983). Dikaryotic and monokaryotic mycelia have been shown to produce asexual spores in some species (Miller 1971; Nakasone 1990), but asexual forms are apparently not as widespread as in the Ascomycota.
Mushrooms are multicellular fruiting bodies produced under favorable conditions by dikaryotic mycelia. Sometimes, mushrooms are produced at the periphery of a circular mycelium, resulting in a fairy ring (Fig. 2). Fairy rings indicate the spatial extent of the mycelium, which is otherwise difficult to establish.


Figure 2. Fairy ring. © Darrell D. Hensley. From the University of Tennessee Turfgrass Diseases page; used with permission.
Nuclear fusion (karyogamy) and meiosis occur in basidia, which are formed in a layer of cells called the hymenium. In addition to basidia, the hymenium often includes specialized non-reproductive cells called cystidia. Cystidia and other anatomical features of mushrooms provide many characters used in Agaricomycete taxonomy (Clémençon 1997; Donk 1964; Singer 1986).
Most groups of Agaricomycetes produce undivided basidia, called homobasidia (Fig. 3), but other members of the Agaricomycetes produce basidia that are divided by septa, including the Auriculariales, Sebacinales, and certain members of the Cantharellales (Tulasnella). Most of the fungi now classified in the Agaricomycetes used to be placed in the "Homobasidiomycetes" (Hibbett and Thorn 2001), but that name has been abandoned in recognition of the fact that not all members of the group have homobasidia.
Two to eight basidiospores (meiospores) are formed on each basidium—the most common number of spores is four. Most textbook illustrations of spore production in Agaricomycetes show four meiotic products migrating into four spores, leaving behind an empty basidium. The actual events of basidiosporogenesis are more complicated, however (Fig. 3). Basidiosporogenesis usually involves a post-meiotic mitosis, yielding eight haploid nuclei (as in Ascomycota). The post-meiotic mitosis may occur in the basidia, spores, or sterigmata (the stalks on which the spores are produced). If it occurs in the spores, then there may or may not be a back-migration of the "extra" nuclei into the basidia. Thus, there are diverse patterns of nuclear behavior leading to the production of spores. Variations in these patterns might provide clues to phylogenetic relationships in Agaricomycetes, but they have been studied in too few species to make broad generalizations (Hibbett et al 1994a, Mueller and Ammirati 1993).

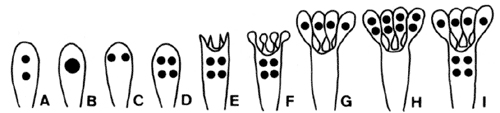
Figure 3. Basidiosporogenesis. A: Pre-fusion dikaryotic stage. B-D: Post-fusion diploid nucleus and meiosis. E-G: Spore development and nuclear migration into spores. H: Post-meiotic mitosis. I: Back-migration of nuclei into the basidium. © Mycological Society of America. From Hasebe et al. (1991); used with permission.
Although much attention in Agaricomycetes has been focused on fruiting bodies, the mycelium also forms other specialized multicellular structures. Examples include rootlike rhizomorphs, which enable Agaricomycetes to forage along the forest floor (Fig. 4), and sclerotia, which are resting structures from which fruiting bodies may be formed.
Figure 4. Rhizomorphs of Armillaria in culture (left; © James B. Anderson; from the Mycological Society of America Slide Collection) and in nature (right; © American Phytopathological Society; from the APS Education Center Illustrated Glossary). Images used with permission.
Fruiting Body Forms
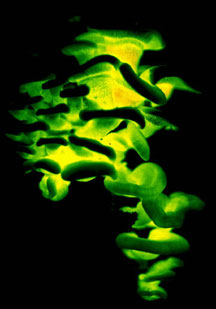
Mushrooms come in many shapes, including coralloid forms, bracket fungi, puffballs, and crustlike resupinate forms (Figs. 5-6). Gross morphology of fruiting bodies was the basis for the nineteenth-century classification of Agaricomycetes and other Fungi by Elias Fries (1874). The Friesian taxa are no longer regarded as natural entities (monophyletic groups, also called clades), but these groupings remain useful for categorizing fruiting body forms and they are emphasized in many useful field guides (e.g., Barron 1999; Bessette et al. 1997; Phillips 1991). Fries made a basic distinction between those fungi that produce their spores internally, which he called Gasteromycetes, and those that produce their spores externally, which he called Hymenomycetes. Gasteroid forms are now understood to have evolved repeatedly from hymenomycetes (note the lower-case "h", indicating that this is an informal, descriptive term referring to mushrooms with external spore-bearing structures, rather than a formal name for a taxon). The most common gasteroid forms are puffballs (Fig. 5) and false-truffles (tuberlike fruiting bodies that are formed underground). Puffballs and false truffles have evolved independently in multiple clades of Agaricomycetes. Uniquely-evolved gasteroid forms include bird's nest fungi, stinkhorns, earthstars (see the title illustration), and the "cannon-ball fungus", Sphaerobolus stellatus (Brodie 1975, Coker and Couch 1928, Miller and Miller 1988).
In some hymenomycetes, the hymenium is initially formed inside the fruiting body, but later becomes exposed as the cap expands (Fig. 5). It has been suggested that a developmental arrest in such forms could lead to the evolution of gasteroid forms (Bruns et al 1989; Hibbett et al. 1994b, Thiers 1984).
Figure 5. The hymenium in the hymenomycete Agaricus (left, © D. Hibbett) is initially concealed by veils. Several groups of puffballs, including the giant puffball Calvatia (right, © M. Binder), are closely related to Agaricus and may have been derived by paedomorphosis.
The Friesian classification divided the "Hymenomycetes" according to the configuration of the spore-bearing surfaces (the hymenophore). Thus, all gilled forms were placed in the Agaricaceae (Fig. 5, left), all poroid forms were placed in the Polyporaceae, all toothed forms were placed in the Hydnaceae, and so on. The taxa just named are still recognized in modern taxonomy, but in much more limited scope than the original Friesian concepts. It is now well understood that the hymenophore types that Fries used to define his taxa have evolved over and over (Hibbett 2007). Thus, there are non-gilled forms in the Agaricaceae (Fig. 5), as well as many gilled forms outside of the Agaricaeae. Commonly used terms like agaricoid, polyporoid, hydnoid, and gasteroid, are derived from the names of the Friesian taxa, but they are purely descriptive and refer to polyphyletic groups (Fig. 6).
  |
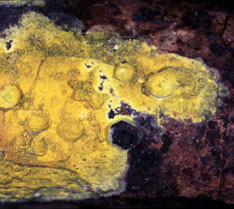  |
Figure 6. Fruiting body diversity in Agaricomycetes. From top left: Auriscalpium vulgare (Russulales), with a toothed or hydnoid hymenophore © Taylor F. Lockwood; Fomitopsis pinicola (Polyporales), a poroid bracket fungus © Mike Wood; Phlebia chrysocrea (Polyporales), a resupinate form © D. Hibbett, Ramaria botrytis (Phallomycetidae), a coralloid form © Taylor F. Lockwood. The Auriscalpium, Fomitopsis and Ramaria images are from MykoWeb and FUNGIPHOTO.COM; used with permission.
Discussion of Phylogenetic Relationships
Understanding of the phylogenetic relationships of the Agaricomycetes has improved dramatically since the mid-1990s. As in all Fungi, the first wave of phylogenetic studies in Agaricomycetes was based on rRNA genes, following the description of conserved primer sequences for PCR amplification (White et al. 1990). This early work was summarized by Hibbett and Thorn (2001), who recognized eight major clades, labelled with informal names, such as “euagarics clade”, “russuloid clade”, “polyporoid clade”, etc.
One of the most commonly-sampled regions in Agaricomycete phylogenetics has been the nuclear-encoded large subunit (nuc-lsu) rRNA gene. At present, the most inclusive published trees are based on the nuc-lsu rRNA, including studies by Moncalvo et al. (2002), with 877 taxa, and Binder et al. (2005), with 656 taxa. An on-line project in automated phylogenetics of Agaricomycetes has generated trees with almost 3000 sequences, and this dataset is being automatically updated with new records from Genbank (Hibbett et al. 2005; http://mor.clarku.edu/).
While the nuc-lsu rRNA studies have been instrumental in fleshing out the sampling of Agaricomycetes, it has long been clear that these genes do not provide robust resolution of many of the deeper nodes. In an attempt to improve resolution of the major groups, Binder and Hibbett (2002) combined data from small and large subunits of nuclear and mitochondrial rDNA (3.8 kilobases per species) in 93 species that span much of the diversity of Agaricomycetes. This analysis provided strong support for seven of the eight major groups that Hibbett and Thorn (2001) proposed, and also suggested some novel relationships, such as a sister-group relationship between Agaricales and Boletales. Nevertheless, the deeper relationships among the major clades were poorly resolved, and the “polyporoid clade” remained weakly supported and controversial (Larsson et al. 2004).
Recently, analyses combining rRNA genes with protein-coding genes have begun to appear, and these have provided great improvements in resolution and support (Matheny et al. 2006, 2007). For example, the “polyporoid clade” has been strongly supported as monophyletic (Matheny et al. 2007).
The current classification of the Agaricomycetes is based on a combination of rRNA and combined protein/rRNA studies, which are too numerous to review here. For a compilation of the phylogenetic studies that inform the current classification, see Hibbett et al. (2007). The informal names of Hibbett and Thorn (2001) have now been replaced by formal taxonomic names, most of which were preexisting in the taxonomic literature. Thus, the current concept of Agaricales is equivalent to the “euagarics clade”, the Polyporales is equivalent to the “polyporoid clade”, the Phallomycetidae is equivalent to the “gomphoid-phalloid clade”, and so on.
There have been several independent clades discovered in recent years that were not included in Hibbett and Thorn’s (2001) overview. These include clades composed mostly of resupinate forms (Corticiales, Trechisporales, and Atheliales), as well as the “heterobasidiomycetous” Sebacinales, which includes coralloid, resupinate, and encrusting forms (Hibbett and Binder 2001; Larsson 2002; Lim 2001; Langer 2002; Weiss et al. 2004). With further study of the cryptic resupinate forms, it is entirely possible that additional major groups of Agaricomycetes will be discovered.
In the tree at the top of this page, the deepest “backbone” nodes in the Agaricomycetes are drawn as a large polytomy, reflecting lingering uncertainty about their resolution (this is a somewhat conservative view, because the multi-locus studies suggest some resolution; see Matheny et al. [2007]). Resolving the earliest divergences in the Agaricomycetes is of interest, because this could provide insight into the form and ecology of the common ancestor of Agaricomycetes. Molecular data do not yet provide robust resolution of this problem, but ultrastructure of parenthesomes may provide some clues. Most Agaricomycetes have perforate parenthesomes, but the Cantharellales, Phallomycetidae, Hymenochaetales, and Trechisporales include species with imperforate parenthesomes. The Auriculariales and Dacrymycetes (see the Agaricomycotina page) also have imperforate parenthesomes, which may therefore be the plesiomorphic condition in the Agaricomycetes. Consistent with this view, the Cantharellales and Phallomycetidae clade have frequently been resolved as basal clades in the Agaricomycetes, albeit with weak bootstrap support (Hibbett and Binder 2002; Binder and Hibbett 2002; Matheny et al. 2007). Several authors have suggested that there is homoplasy in the evolution of parenthesomes in Agaricomycetes (Larsson 2002; Hibbett and Thorn 2001).
In sum, there are nineteen major clades of Agaricomycetes that are formally named in the current classification. The tree shown here also includes a few nodes that are resolved but are not named. Support for these groupings is moderate to strong in some analyses, and in the future these clades may also be recognized as formal taxa (if they are not refuted!).
References
Alexopoulos, C.J., Mims, C.W. and Blackwell, M. 1996. Introductory Mycology. John Wiley and Sons, New York.
Anderson, J. B. 1983. Induced somatic segregation in Armillaria mellea diploids. Exp. Mycol. 7:141-147.
Barron, G. 1999. Mushrooms of Northeast North America. Lone Pine Publishing, Edmonton.
Bessette, A. E., Bessette, A. R., and Fischer, D. W. 1997. Mushrooms of Northeastern north America. Syracuse University Press, Syracuse.
Binder, M., and D. S. Hibbett. 2002. Higher-level phylogenetic relationships of homobasidiomycetes (mushroom-forming fungi) inferred from four rDNA regions. Molecular Phylogenetics and Evolution 22: 76-90.
Blanchette, R. A., Compton, B. D., Turner, N. J., and Gilbertson, R. L. 1992. Nineteenth century shaman grave guardians are carved Fomitopsis officinalis sporophores. Mycologia 84:119-124.
Brodie, H.J. 1975. The Bird's Nest Fungi. University of Toronto Press, Toronto.
Bruns TD, Fogel R, White TJ, Palmer JD. 1989. Accelerated evolution of a false truffle from a mushroom ancestor. Nature 339:140-142
Burdsall, H. H., Jr., Volk, T. J., and Ammirati, J. F., Jr. 1996. Bridgeoporus, a new genus to accommodate Oxyporus nobilissimus (Basidiomycotina, Polyporaceae). Mycotaxon 60:387-395.
Capasso, L. 1998. 5300 years ago, the Ice Man used natural laxatives and antibiotics. The Lancet 352:1864.
Clémençon, H. 1997. Anatomie der Hymenomyceten. F. Flück-Wirth, Teufen.
Coker, W. C. and Couch, J. N. 1928. The Gasteromycetes of the eastern United States and Canada. University of North Carolina Press, Chapel Hill.
Donk, M. A. 1964. A conspectus of the families of the Aphyllophorales. Persoonia 3:199-324.
Hasebe, K., Murakami, S., and Tsuneda, A. 1991. Cytology and genetics of a sporeless mutant of Lentinus edodes. Mycologia 83:354-359.
Fries, E. M. 1874. Hymenomycetes Europaei. Upsaliae.
Hibbett, D. S. 2006. A Phylogenetic overview of the Agaricomycotina. Mycologia 98: 917-925.
Hibbett, D. S. 2007. After the gold rush, or before the flood? Evolutionary morphology of mushroom-forming fungi (Agaricomycetes) in the early 21st century. Mycological Research in press.
Hibbett DS, Binder M. 2002. Evolution of complex fruiting body morphologies in homobasidiomycetes. Proc Roy Soc London Ser B 269:1963—1969.
Hibbett, D. S., M. Binder, J. F. Bischoff, M. Blackwell, P. F. Cannon, O. E. Eriksson, S. Huhndorf, T. James, P. M. Kirk, R. Lücking, T. Lumbsch, F. Lutzoni, P. B. Matheny, D. J. Mclaughlin, M. J. Powell, S. Redhead, C. L. Schoch, J. W. Spatafora, J. A. Stalpers, R. Vilgalys, M. C. Aime, A. Aptroot, R. Bauer, D. Begerow, G. L. Benny, L. A. Castlebury, P. W. Crous, Y.-C. Dai, W. Gams, D. M. Geiser, G. W. Griffith, C. Gueidan, D. L. Hawksworth, G. Hestmark, K. Hosaka, R. A. Humber, K. Hyde, J. E. Ironside, U. Kõljalg, C. P. Kurtzman, K.-H. Larsson, R. Lichtwardt, J. Longcore, J. Miądlikowska, A. Miller, J.-M. Moncalvo, S. Mozley-Standridge, F. Oberwinkler, E. Parmasto, V. Reeb, J. D. Rogers, C. Roux, L. Ryvarden, J. P. Sampaio, A. Schüßler, J. Sugiyama, R. G. Thorn, L. Tibell, W. A. Untereiner, C. Walker, Z. Wang, A. Weir, M. Weiß, M. M. White, K. Winka, Y.-J. Yao, and N. Zhang. 2007. A higher-level phylogenetic classification of the Fungi. Mycological Research 111: 509-547.
Hibbett, D. S., Murakami, S. and Tsuneda, A. 1994a. Postmeiotic nuclear behavior in Lentinus, Panus, and Neolentinus. Mycologia 86:725-732.
Hibbett, D. S., R. H. Nilsson, M. Snyder, M. Fonseca, J. Costanzo, and M. Shonfeld. 2005. Automated Phylogenetic Taxonomy: An Example in the Homobasidiomycetes (Mushroom-Forming Fungi). Systematic Biology 54: 660-668.
Hibbett, D. S., Pine, E. M., Langer, E., Langer, G. and Donoghue, M. J. 1997. Evolution of gilled mushrooms and puffballs inferred from ribosomal DNA sequences. Proc Nat Acad Sci USA 94:12002-12006.
Hibbett, D. S., and R. G. Thorn. 2001. Basidiomycota: Homobasidiomycetes. Pp. 121-168 in: The Mycota, vol. VII part B, Systematics and Evolution (D. J. McLaughlin, E. G. McLaughlin, and P. A. Lemke, eds.). Springer Verlag.
Hibbett DS, Tsuneda A, Murakami S (1994b) The secotioid form of Lentinus tigrinus: genetics and development of a fungal morphological innovation. Am J Bot 81:466-478.
Langer E. 2002. Phylogeny of non-gilled and gilled basidiomycetes: DNA sequence inference, ultrastructure and comparative morphology. Habilitationschrift, Tübingen University, Tübingen, Germany.
Larsson, E. 2002. Phylogenetic corticioid fungi with russuloid characteristics. PhD Thesis, Göteborg University, Göteborg, Sweden. 134 p.
Lim YW. 2001. Systematic study of corticioid fungi based on molecular sequence analyses. PhD Thesis, School of Biological Sciences, Seoul National University, Korea. 228p.
Matheny, P. B., Z. Wang, M. Binder, J. M. Curtis, Y. W. Lim. R. H. Nilsson, K. W. Hughes, R. H. Petersen, V. Hofstetter, J. F. Ammirati, C. Schoch, G. E. Langer, D. J. McLaughlin, A. W. Wilson, P. E. Crane, T. Frøslev, Z. W. Ge, R. W. Kerrigan, J. C. Slot, E. C. Vellinga, Z. L. Liang, M. C. Aime, T. J. Baroni, M. Fischer, K. Hosaka, K. Matsuura, M. T. Seidl, J. Vaura, and D. S. Hibbett. 2007. Contributions of rpb2 and tef1 to the phylogeny of mushrooms and allies (Basidiomycota, Fungi). Molecular Phylogenetics and Evolution in press.
Miller, O. K., Jr. 1971. The relationship of cultural characters to the taxonomy of the agarics In: Petersen RH (ed) Evolution in the higher basidiomycetes. University of Tennessee Press, Knoxville, pp 197-216.
Miller, O. K., Jr. and Miller, H. 1988. Gasteromycetes: morphological and developmental features. Mad River Press, Eureka.
Moncalvo JM, Vilgalys R, Redhead SA, Johnson JE, James TY, Aime MC, Hofstetter V, Verduin SJW, Larsson E, Baroni TJ, Thorn RG, Jacobsson S, Clémençon H, Miller OK Jr. 2002. One Hundred and Seventeen Clades of Euagarics. Mol Phyl Evol 23:357—400.
Mueller, G. M. and Ammirati, J. F. 1993. Cytological studies in Laccaria (Agaricales). II. Assessing phylogenetic relationships among Laccaria, Hydnangium, and other Agaricales. Am J Bot 80:322-329.
Mueller, U. G., S. A. Rehner, and T. R. Schultz. 1998. The evolution of agriculture in ants. Science 281:2034-2038.
Nakasone, K. K. 1990. Cultural studies and identification of wood-inhabiting Corticiaceae and selected Hymenomycetes from North America. Mycol. Memoir 15:1-412
Patouillard, N. T. 1900. Essai taxonomique sur les familles et les genres des Hyménomycètes. Declume, Lons-le-Saunier. 184p.
Pegler, D. N., Læssøe, T, Spooner, B. M. 1995. British puffballs, earthstars and stinkhorns. Royal Botanic Gardens, Kew.
Phillips, R. 1991. Mushrooms of North America. Little, Brown and Company, Boston.
Raper, J. R. and Flexer, A. S. 1971. Mating systems and evolution of the basidiomycetes. In: Petersen RH (ed) Evolution in the Higher Basidiomycetes. University of Tennessee Press, Knoxville, pp 149-168.
Rayner, A. D. M. and Boddy, L. 1988. Fungal decomposition of wood: its biology and ecology. John Wiley and Sons, Chichester.
Singer, R. 1986. The Agaricales in modern taxonomy. 4th ed. Koeltz Scientific Books, Koenigstein.
Smith , M., Bruhn, J., and Anderson, J. 1992. The fungus Armillaria bulbosa is among the largest and oldest living organisms. Nature 356:428-431.
Thiers, H. D. 1984. The secotioid syndrome. Mycologia 76:1-8.
Smith, S. E. and Read, D. J. 1997. Mycorrhizal symbiosis. Academic Press, San Diego.
Weiß M, Bauer R, Begerow D, 2004. Spotlights on heterobasidiomycetes. In: Agerer R, Piepenbring M, Blanz P, (eds),. Frontiers in basidiomycote Basidiomycote mycology. IHW Verlag, Eching, pp. 7-48.
Weiß, M. and Oberwinkler, F. O. 2001. Phylogenetic relationships in Auriculariales and related groups-hypotheses derived from nuclear ribosomal DNA sequences. Mycol. Res. 105:403-415.
Weiß M, Selosse M-A, Rexer K-H, Urban A, Oberwinkler O, 2004. Sebacinales: a hitherto overlooked cosm of heterobasidiomycetes with a broad mycorrhizal potential. Mycological Research 108: 1003-1010.
White TJ, Bruns TD, Lee S, Taylor JW, 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky J, White TJ (, eds),. PCR Pprotocols:, a guide to methods and applications,. Academic Press, San Diego, pp. 315-322.
Information on the Internet
- Mycological Society of America. Extensive links to sites concerned with Basidiomycota and other fungi.
- The WWW Virtual Library: mycology.
- Deep Hypha Research Coordination Network. Deep Hypha is a project to coordinate and provide resources for research in fungal systematics.
- AFTOL: Assembling the Fungal Tree of Life. Collaborative research in fungal phylogenetics.
- mor. On-line automated molecular phylogenetic taxonomy of Agaricomycetes.
- Bridgeoporus nobilissimus and Armillaria gallica have both been featured as the “Fungus of the Month” at Tom Volk's Fungi.
- Australian National Botanic Gardens - Fungi Web Site. Gasteromycetes page. Excellent overview of the morphology and antomy of these strange fungi.
- MykoWeb - California Mushrooms. The first level in the "simple key" is organized according to gross morphology of fruiting bodies, with illustrations. This is a good place to get an overview of fruiting body diversity in Agaricomycetes and other fleshy Fungi.
Title Illustrations

| Scientific Name | Lepista cf. nuda |
|---|---|
| Comments | Agaricales |
| Image Use |
 This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0. This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0.
|
| Copyright |
© 2003 David S. Hibbett

|
| Scientific Name | Geastrum saccatum |
|---|---|
| Comments | Earthstar (Geastrales) |
| Image Use |
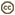 This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0. This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0.
|
| Copyright |
© 2003 David S. Hibbett

|
About This Page
Development of this page was facilitated by the "Deep Hypha" Research Coordination Network and the "Assembling the Fungal Tree of Life" project (NSF awards DEB-0090301 and DEB-0228657). Many thanks to Jim Anderson, Manfred Binder, Darrel Hensley, Taylor Lockwood, Mike Wood, the American Phytopathological Society, and the Mycological Society of America for permission to reproduce the images in Figs. 1-7, and Dan Dvorak for helpful comments.
David S. Hibbett

Clark University, Worcester, Massachusetts, USA
Correspondence regarding this page should be directed to David S. Hibbett at
Page copyright © 2003 David S. Hibbett
 Page: Tree of Life
Agaricomycetes. Mushroom-Forming Fungi.
Authored by
David S. Hibbett.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Agaricomycetes. Mushroom-Forming Fungi.
Authored by
David S. Hibbett.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
- First online 24 October 2003
- Content changed 20 April 2007
Citing this page:
Hibbett, David S. 2007. Agaricomycetes. Mushroom-Forming Fungi. Version 20 April 2007. http://tolweb.org/Agaricomycetes/20535/2007.04.20 in The Tree of Life Web Project, http://tolweb.org/














 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site