Anura
David Cannatella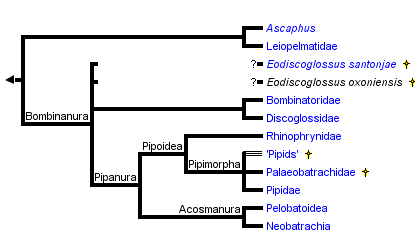


This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
Anura is the clade of living frogs and their close fossil relatives. The name Anura refers to the lack of a tail.
Characteristics
When only living taxa are considered, a list of synapomorphies of Anura would include many of the obvious distinctive features of frogs:
- Shortened vertebral column (nine or fewer presacral vertebrae)
- Presence of a urostyle formed from developing tail vertebrae
- Absence of tail in adults
- Hindlimb longer than forelimb
- Fusion of radius and ulna into a single element
- Fusion of tibia and fibula into a single element
- Elongate ankle bones (tibiale and fibulare = astragalus and calcaneum)
- Absence of a prefrontal bone
- Fusion of separate hyobranchial elements into a hyobranchial (=hyoid) plate
- A tongue that lacks intrinsic skeletal support from the hyobranchial plate
- A tadpole, with keratinous beaks and denticles as larval mouthparts
- A single median spiracle in the larva (characteristic of Orton's Type 3 tadpole)
- Large subcutaneous lymph spaces between the skin and muscle layer
- Two protractor lentis muscles attached to lens
(Milner, 1988, 1993; Saint-Aubain, 1981; Trueb and Cloutier, 1991).
However, the existence of several early frog fossils, such as †Prosalirus and †Notobatrachus, clouds this picture, because many of these characters cannot be assessed in these fossils, either because of incompleteness of fossils or because the features are soft-tissue characters identifiable only in living taxa.
Discussion of Phylogenetic Relationships
The node-based name Anura was defined by Ford and Cannatella (1993) as the last common ancestor of living frogs and all its descendants. According to this definition, †Triadobatrachus is not part of Anura, following Trueb and Cloutier (1991). The late Jurassic fossil †Notobatrachus degiustoi has been considered as having uncertain placement with respect to Anura--possibly the sister-group, possibly not (Cannatella, 1985); or related to Leiopelma (Estes and Reig, 1973). Baez and Basso (1996) placed it as the sister-group of Anura.
†Vieraella herbstii is another relatively complete early Jurassic fossil, but is less well-preserved than †Notobatrachus (Estes and Reig, 1973). The presence of nine presacral vertebrae places it among the basal salientians, but other characters are not sufficiently preserved to permit definitive placement in Anura. A third well-preserved Jurassic fossil taxon, †Eodiscoglossus santonje, has eight presacral vertebrae (Estes and Reig, 1973), and thus is clearly within Anura. †Prosalirus bitis is from the Early Jurassic (Pliensbachian) of Arizona; the putative limbed caecilian †Eocaecilia is from the same formation. It is reputed to be the earliest known "frog" in the sense that it has froglike features such as a urostyle and elongate, anteriorly directed ilia. For more details see the leaf page on †Prosalirus.
The monophyly of Anura has rarely been questioned. Griffiths (1963:279) considered Anura to be diphyletic; i.e., Ascaphus and Leiopelma comprised one lineage, and all other frogs a second, "...stemming independently from either different levels of a single proanuran organization or different proanuran stocks." Rocek (1981) considered †Triadobatrachus to be within Anura, and placed Pelobatidae (including only Pelobates and some related fossils), and somewhat tentatively, Pipidae, †Palaeobatrachidae, and Rhinophrynidae, in Archaeosalientia. †Triadobatrachus and remaining anurans were included in Neosalientia. The character distinguishing the two groups is a median dermal bone, the interparietal (Reinbach, 1939), which Rocek homologized with the median extrascapular of the osteolepiform fish †Eusthenopteron. The interparietal is found in Pelobates and some fossil relatives, but not in any pipoid frogs. Milner (1988) provided a cogent review of the interpretation of this dermal element.
In a study of tetrapod phylogeny, Hedges et al. (1990) analyzed 123 phylogenetically informative sites from 18S ribosomal RNA of 21 tetrapods, including 4 salamanders, 4 caecilians, and 1 species each from Bufonidae, Discoglossidae, Hylidae, Leptodactylidae, Microhylidae, Pelobatidae, and Sooglossidae. Bootstrap analyses using both maximum-parsimony and neighbor-joining algorithms did not support the monophyly of Anura, Caudata, or Gymnophiona, but a monophyletic Amphibia was supported with a bootstrap value of 100%. Hedges and Maxson (1993) and Hillis et al. (1993) presented analyses of anuran relationships based on DNA sequence data from the mitochondrial and nuclear ribosomal genes, respectively.
Hedges et al. (1990) also analyzed 35 variable sites from 28S rRNA of four species of frogs, from Discoglossidae, Hylidae, Pelobatidae, and Pipidae. Bootstrap analysis of maximum parsimony trees yielded a monophyletic Anura, but with no resolution among the four taxa. The neighbor-joining analysis indicated a sister-group relationship between the discoglossid and pipid, and between the bufonid and hylid, but the bootstrap value for both of these nodes was less than 50%. Neither analysis yielded a monophyletic Amphibia.
Hay et al. (1995) is the most comprehensive molecular systematics treatment of the relationships among the families of amphibians. Their neighbor-joining tree yielded a monophlyletic Anura, Caudata, and Gymnophiona.
"Archaeobatrachia"
Archaeobatrachians have generally included discoglossoids, pipoids and pelobatoids (Duellman, 1975; Reig, 1958). As discussed on other pages, the synapomorphies of Bombinanura and Pipanura collectively demonstrate that "Archaeobatrachia" is paraphyletic. The informal term archaeobatrachian is a convenient term for anurans that are not part of Neobatrachia.
Bombinanura
Bombinanura, a node-based name, was defined by Ford and Cannatella (1993) to be the most recent common ancestor of living Bombinatoridae and Discoglossanura, and all its descendants. Synapomorphies of Bombinanura include fusion of the halves of the sphenethmoid, eight presacral vertebrae, absence of the m. epipubicus, and absence of the caudalipuboischiotibialis muscle (Cannatella, 1985). Subclades of Bombinanura include Bombinatoridae and Discoglossanura.
Discoglossidae and Discoglossoidea
Most analyses of molecular sequence data have placed Alytes, Discoglossus, Bombina, and Barbourula in a clade, to which the name Discoglossidae has been applied. In contrast, Ford and Cannatella (1993) listed derived morphological features that would require paraphyly of this group , and they recognized both Discoglossidae (for Alytes and Discoglossus) and Bombinatoridae (for Bombina and Barbourula). A synthesis of the two datasets has not been attempted. There are apparently no published synapomorphies for "Discoglossidae," and the dissimilarity of Alytes and Discoglossus, on one hand, and Bombina on the other, has often been noted (e.g., Lanza et al., 1976), suggesting a deep divergence between the two lineages. Griffiths (1963) stated that the diagnostic feature of Discoglossidae (sensu lato) was a triradiate sternum. However, this feature is also present in Leiopelma. The similarity can be interpreted either as shared plesiomorphy between Leiopelma and Discoglossidae, or a derived feature that unites Leiopelma more closely to Discoglossidae than to Ascaphus.
Pipanura
The node-based name Pipanura was proposed by Ford and Cannatella (1993) for the most recent common ancestor of living Mesobatrachia + Neobatrachia, and all its descendants. The subordinal name Ranoidei was coined for this clade by Sokol (1977), but that name was re-assigned to a less inclusive taxon by Dubois (1983, 1984). Sokol's (1977) use of this name was unfortunate because the informal name, ranoid, is homonymous with the widely used name Ranoidea.
Synapomorphies include a sinistral spiracle in the larvae (a characteristic feature of Orton's Type 4 tadpole), absence of free ribs in adults, torsion in the carpal elements, the presence of vocal sacs, and fusion of the trigeminal and facial ganglia (Cannatella, 1985; Sokol, 1975).
Acosmanura
Pelobatoidea
The name was applied to the node that is the common ancestor of living Megophryidae, Pelobatidae, and Pelodytes. Synapomorphies of Pelobatoidea include the presence of a palatine process of the maxilla and ossification of the sternum into a bony style (Cannatella, 1985). Duellman and Trueb (1986) listed the presence of a dorsal gap in the cricoid ring as a synapomorphy for this clade. However, there is no gap in Scaphiopus, Spea, Pelobates (except for the smallest species, P. fuscus), or several megophryids; at best, the presence of a dorsal gap would be an ambiguous synapomorphy. Relationships among the living pelobatoids are an unresolved trichotomy of Megophryidae, Pelobatidae, and Pelodytes.
Pipoidea
Pipoidea was defined by Cannatella and Ford (1993) to be the most recent common ancestor of living Pipidae + Rhinophrynidae, and all its descendants. Pipoidea is diagnosed by several distinctive synapomorphies, including the absence of mentomeckelian bones (see comments below on †Palaeobatrachidae), absence of lateral alae of the parasphenoid, fusion of the frontoparietals into an azygous element, greatly enlarged otic capsules, and a tadpole with paired spiracles, and lacking beaks and denticles (Orton Type 1 tadpole). Clades of Pipoidea include Pipidae, †Palaeobatrachidae, some unplaced fossil "pipids," and Rhinophrynidae.
Pipimorpha
The new stem-based name Pipimorpha was defined by Ford and Cannatella (1993) to be those taxa that are more closely related to living Pipidae than to living Rhinophrynus. Pending a more detailed assessment of the relationships of †Palaeobatrachidae, Pipidae, and the fossil "pipids," we consider †Palaeobatrachidae, †Thoraciliacus, †Cordicephalus, †Saltenia, †Shomronella, and †Eoxenopoides to be part of Pipimorpha.
"Mesobatrachia"
Ford and Cannatella (1993) applied the name Mesobatrachia to the node that is the most recent common ancestor of the living Pelobatoidea and Pipoidea. This relationship was based on synapomorphies discussed by Cannatella (1985). Mesobatrachia as proposed by Laurent (1979) was a paraphyletic group. Cannatella (1985) first applied the name to a clade. Synapomorphies reported for Mesobatrachia included closure of the frontoparietal fontanelle by juxtaposition of the frontoparietal bones, partial closure of the hyoglossal sinus by the ceratohyals, absence of the taenia tecti medialis, and absence of the taenia tecti transversum (Cannatella, 1985; Sokol, 1981).Most other previous taxonomies, and recent molecular phylogenetic analyses, have placed the Pelobatoidea as the sister-group to Neobatrachia, rather than to Pipoidea. Thus, the Mesobatrachia as formerly proposed is not monophyletic, and therefore no longer recognized.
References
Báez, A. M., and N. G. Basso. 1996. The earliest known frogs of the Jurassic of South America: review and cladistic appraisal of their relationships. Münchner Geowiss. Abh. (a) 30:131-158.
Cannatella, D. C. 1985. A phylogeny of primitive frogs (archaeobatrachians). Ph.D. Dissertation, The University of Kansas, Lawrence.
Cannatella, D. C., and D. M. Hillis. 1993. Amphibian phylogeny: phylogenetic analysis of morphology and molecules. Herpetol. Monogr. 7:1-7.
Duellman, W. E. 1975. On the classification of frogs. Occ. Pap. Mus. Nat. Hist. Univ. Kansas (42):1-15.
Estes, R., and O. A. Reig. 1973. The early fossil record of frogs: a review of the evidence. Pp. 11-63 In J. L. Vial (Ed.), Evolutionary Biology of the Anurans: Contemporary Research on Major Problems. University of Missouri Press, Columbia.
Ford, L. S., and D. C. Cannatella. 1993. The major clades of frogs. Herp. Monogr. 7:94-117.
Griffiths, I. 1963. The phylogeny of the Salientia. Biological Reviews 38:241-292.
Hay, J. M., I. Ruvinsky, S. B. Hedges, and L. R. Maxson. 1995. Phylogenetic relationships of amphibian families inferred from DNA sequences of mitochondrial 12S and 16S ribosomal RNA genes. Mol. Biol. Evol. 12(5):928-937.
Hedges, S. B., K. D. Moberg, and L. R. Maxson. 1990. Tetrapod phylogeny inferred from 18s and 28s ribosomal RNA sequences and a review of the evidence for amniote relationships. Mol. Biol. Evol. 7:607-633.
Hedges, S. B. and L. R. Maxson. 1993. A molecular perspective on lissamphibian phylogeny. Herpetol. Monogr. 7:27-42.
Hillis, D. M., L. K. Ammerman, M. T. Dixon, and R. O. de Sá. 1993. Ribosomal DNA and the phylogeny of frogs. Herpetol. Monogr. 7:118-131.
Reig, O. A. 1958. Proposiciones para una nueva macrosistematica de los anuros. Nota preliminar. Physis 21:109-118.
Rocek, Z. 1981. Cranial anatomy of frogs of the family Pelobatidae Stannius, 1856, with outlines of their phylogeny and systematics. Acta Univ. Carolinae Biol. 1980:1-164.
Title Illustrations

| Scientific Name | Bombina bombina |
|---|---|
| Location | Romania |
| Specimen Condition | Live Specimen |
| Identified By | Horia Bogdan |
| Copyright |
© Horia Bogdan

|
| Scientific Name | Agalychnis callidryas |
|---|---|
| Location | Costa Rica |
| Specimen Condition | Live Specimen |
| Source | Red-Eyed Treefrog |
| Source Collection | Flickr |
| Image Use |
 This media file is licensed under the Creative Commons Attribution-NonCommercial-ShareAlike License - Version 2.0. This media file is licensed under the Creative Commons Attribution-NonCommercial-ShareAlike License - Version 2.0.
|
| Copyright | © 2006 Jack G |
About This Page
David Cannatella

University of Texas, Austin, Texas, USA
Correspondence regarding this page should be directed to David Cannatella at
Page copyright © 2008 David Cannatella
 Page: Tree of Life
Anura.
Authored by
David Cannatella.
The TEXT of this page is licensed under the
Creative Commons Attribution License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Anura.
Authored by
David Cannatella.
The TEXT of this page is licensed under the
Creative Commons Attribution License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
- First online 11 January 2008
- Content changed 11 January 2008
Citing this page:
Cannatella, David. 2008. Anura. Version 11 January 2008 (under construction). http://tolweb.org/Anura/16963/2008.01.11 in The Tree of Life Web Project, http://tolweb.org/









 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site