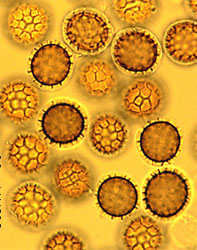
Basidiomycota
The Club Fungi
Eric Swann and David S. Hibbett

This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxThe Basidiomycota is the sister group of the Ascomycota. The tree shown above is based on Swann and Taylor’s (1993) analysis of nuclear small subunit ribosomal DNA sequences.
Introduction
The Basidiomycota contains about 30,000 described species, which is 37% of the described species of true Fungi (Kirk et al. 2001). The most conspicuous and familiar Basidiomycota are those that produce mushrooms, which are sexual reproductive structures. The Basidiomycota also includes yeasts (single-celled forms; Fell et al. 2001) and asexual species. Basidiomycota are found in virtually all terrestrial ecosystems, as well as freshwater and marine habitats (Kohlmeyer and Kohlmeyer, 1979; Hibbett and Binder, 2001).
Basidiomycota have a huge impact on human affairs and ecosystem functioning. Many Basidiomycota obtain nutrition by decaying dead organic matter, including wood and leaf litter. Thus, Basidiomycota play a significant role in the carbon cycle. Unfortunately, Basidiomycota frequently attack the wood in buildings and other structures, which has negative economic consequences for humans.
Symbiotic lifestyles (intimate associations with other living organisms) are well developed in the Basidiomycota. Symbiotic Basidiomycota include important plant pathogens, such as "rusts" (Uredinales) and "smuts" (Ustilaginales), which attack wheat and other crops. Other symbiotic Basidiomycota cause diseases in animals, including humans. Not all symbiotic Basidiomycota cause obvious harm to their partners, however. For example, some Basidiomycota, as well as a handful of Ascomycota, form ectomycorrhizae, which are associations with the roots of vascular plants (principally forest trees such as oaks, pines, dipterocarps, and eucalypts; Smith and Read, 1997). Ectomycorrhizal Basidiomycota help their plant partners obtain mineral nutrients from the soil, and in return they receive sugars that the plants produce through photosynthesis. Other symbiotic Basidiomycota form associations with insects, including leaf-cutter ants, termites, scale insects, woodwasps, and bark beetles (Wheeler and Blackwell, 1984; Mueller et al., 1998).
Humans have found diverse uses for Basidiomycota. Mushrooms, both cultivated and wild, are eaten in many countries. For the untrained, mushroom-hunting is a risky endeavor, because some Basidiomycota produce deadly toxins (Benjamin 1995). The basidiomycete toxin phalloidin (from the mushroom Amanita phalloides) binds actin, which is a component of microfilaments. Fluorescent stains that incorporate phalloidin are used by cell biologists to visualize the cytoskeleton. Other "toxins" produced by Basidiomycota include hallucinogens, which are produced by members of the genus Psilocybe (and other groups). Species of Psilocybe have traditionally been used in Central American indigenous cultures as a spiritual tool, and are now cultivated for the illicit drug trade. Other biochemical compounds of Basidiomycota that have practical uses include astaxanthin, a red pigment produced by the basidiomycetous yeast Phaffia (used to add color to farmed salmon), and certain enzymes from wood-decaying Basidiomycota that have potential applications in paper production and bioremediation (decontamination of polluted environments using biological agents).
Characteristics
Basidiomycota are unicellular or multicellular, sexual or asexual, and terrestrial or aquatic. Indeed, Basidiomycota are so variable that it is impossible to identify any morphological characteristics that are both unique to the group and constant in the group. The most diagnostic feature is the production of basidia (sing. basidium), which are the cells on which sexual spores are produced, and from which the group takes its name. A long-lived dikaryon, in which each cell in the thallus contains two haploid nuclei resulting from a mating event, is another characteristic feature. Finally, clamp connections are a kind of hyphal outgrowth that is unique to Basidiomycota, although they are not present in all Basidiomycota. The following description of the characteristics of Basidiomycota traces the life cycle of a "typical" species, beginning at the site of meiosis.
The basidium is the cell in which karyogamy (nuclear fusion) and meiosis occur, and on which haploid basidiospores are formed (basidia are not produced by asexual Basidiomycota). Many Basidiomycota produce basidia on multicellular fruiting bodies (e.g., mushrooms), but basidia can also be formed directly from yeasts or other single cells. There is a great range of variation in morphology of the basidium, the number of spores formed, and how the spores are borne on the surface of the basidium (Ingold 1991). Typically, four spores are produced on each basidium, at the tips of minute stalks called sterigmata (Fig. 1). Each spore usually contains one or two of the haploid meiotic products.
 image info
image info Figure 1. SEM of the surface of a mushroom gill (Coprinus cinereus: Hymenomycetes) showing several basidia, some with four basidiospores attached.
(From McLaughlin, et al. 1985; used with permission © McLaughlin, Beckett and Yoon 1985.)
One of the most fascinating characteristics of Basidiomycota is the production of forcibly discharged ballistospores (Fig. 2), which are propelled into the air from the sterigma. Ballistospores may be sexual or asexual, and may be produced by basidia, hyphae, yeast cells, or even other ballistospores. This type of spore discharge must have evolved very early in the evolutionary history of the Basidiomycota as it is found in members of the earliest diverging lineages within the group. Ballistospory is associated with forms that disperse their spores directly into the air. Most aquatic Basidiomycota and forms that produce spores inside the fruiting body, such as puffballs, have lost ballistospory.
Ballistospory is associated with the production of a liquid filled "hilar droplet" that forms at the base of the spore, just above its attachment to the sterigma (Fig. 2). Resolving the mechanism of ballistospory has been a longstanding problem in mycology (Buller 1909, 1922; Ingold 1939; McLaughlin et al. 1985; Webster et al. 1984a, b; Yoon and McLaughlin 1986). In a series of studies, reviewed by Money (1998), it has been shown that spore discharge occurs when the hilar droplet fuses with a film of liquid on the surface of the spore. The rapid coalescence of the liquids causes a sudden shift in the center of mass of the spore and contributes to its release from the sterigma. This mechanism has been termed a "surface tension catapult" and it results in spores being discharged with a force of about 25,000 g (Money 1998).
 image info
image info Figure 2. Scanning electron micrograph showing the hilar droplet at the base of a basidiospore (Coprinus cinereus: Hymenomycetes).
(From McLaughlin et al. 1985)
Basidiospores germinate to form hyphae (filaments) or yeast cells that are typically haploid and uninucleate. The hyphae of Basidiomycota are septate. Ultrastructural features of the septa, visible with transmission electron microscopy, have been important in developing phylogenetic hypotheses in Basidiomycota (see Hymenomycetes page).
Mating in Basidiomycota involves fusion of haploid cells, but fusion of the nuclei is usually delayed until the basidia are formed. Thus, the dominant phase of the life cycle in most Basidiomycota is a dikaryon, in which the two nuclei brought together in mating exist side-by-side in each cell (Fig. 3A). Sometimes a dikaryon can donate a nucleus to a uninucleate "monokaryon", resulting in a "di-mon" mating.
Clamp connections are hyphal outgrowths that form when cells in dikaryotic hyphae divide. One of the nuclei divides in the main axis of the hypha, while the other divides into the clamp (Fig. 3B). Septa are formed across each of the mitotic spindles. The apex of the backward-growing monokaryotic clamp cell fuses with the subapical cell, reestablishing the dikaryotic condition (Fig. 3C). All fungi that produce clamp connections are members of the Basidiomycota, but not all Basidiomycota produce clamp connections. The regular formation of clamp connections must have developed early in basidiomycete evolution, because they are found in all the major clades of Basidiomycota.
Sexually reproducing Ascomycota also form dikaryons, although they are not as long-lived as those of Basidiomycota. The clade that includes Ascomycota and Basidiomycota has been called the Dikaryomycotina, reflecting this presumably homologous similarity. Ascomycota produce clamp-like "croziers" at the bases of asci (cells in which meiosis occurs, homologous to basidia). Croziers may be homologous to clamp connections.
 image info
image info Figure 3. Diagram of clamp cell formation: A. Dikaryotic hypha, arrow shows direction of hyphal tip growth.
B. Clamp cell growing backward, nuclei undergoing synchronous division.
C. Mature clamp.
Illustrations © E. Swann 1997.
Discussion of Phylogenetic Relationships
There is strong evidence that the Basidiomycota is monophyletic. Ballistospores, basidia, and clamp connections are present in each of its major subgroups (although not in all species), suggesting a common origin. Non-molecular characters that have been used to recognize major groups within the Basidiomycota include the form of the basidia (shape and septation), ultrastructure of hyphal septa and spindle pole bodies, presence or absence of yeast phases and "spore repetition" (production of spores directly from spores), and cellular carbohydrate composition (McLaughlin et al. 1995; Oberwinkler, 1987; Prillinger et al. 1990, 1991). Sequences of ribosomal genes (rDNA) have played a major role in increasing our understanding of the relationships within Basidiomycota, and have demonstrated that some previously emphasized morphological attributes, such as the form of basidia, are subject to homoplasy (Swann and Taylor 1993, 1995; Swann et al. 1999).
Three major groups are recognized within the Basidiomycota: 1) Urediniomycetes includes rusts (Uredinales) and other taxa (Swann et al. 2001); 2) Ustilaginomycetes includes smuts (Ustilaginales) and others (Bauer et al. 2001); and 3) Hymenomycetes includes mushrooms (Homobasidiomycetes), jelly fungi (Auriculariales, Dacrymycetales, Tremellales) and others (Hibbett and Thorn 2001, Swann and Taylor 1995, Wells and Bandoni 2001). Monophyly of each of these groups has been strongly to moderately supported in phylogenetic analyses of rDNA sequences, especially nuclear small subunit rDNA (Swann and Taylor, 1993, 1995; Swann et al. 1999). Analyses of nuclear large subunit rDNA sequences have provided only weak support for monophyly of the Ustilaginales, however (Bauer et al. 2001). The pattern of relationships among the Urediniomycetes, Ustilaginomycetes, and Hymenomycetes are not well resolved by rDNA sequences. Similarities in the ultrastructure of septal pores and spindle pole bodies (McLaughlin et al. 1995) suggest that Ustilaginales and Hymenomycetes could be sister groups, but not all rDNA phylogenies show this topology. Additional data from multiple genes are probably needed to resolve the deepest divergences in the Basidiomycota.
References
Alexopoulos, C.J., Mims, C.W. and Blackwell, M. 1996. Introductory Mycology. John Wiley and Sons, New York.
Arora, D. 1986. Mushrooms Demystified. Ten Speed Press, Berkeley, California.
Bauer, R., Begerow, D., Oberwinkler, F., Piepenbring, M. and Berbee, M. L 2001. Ustilaginomycetes. Pp. 57-84. In: The Mycota VII. Systematics and Evolution. Part B. (Mclaughlin, D. J., McLaughlin, E. G. and Lemke, P. A., eds.). Springer-Verlag, Berlin.
Benjamin, D.R. 1995. Mushrooms: poisons and panaceas. W.H. Freeman and Company, New York.
Buller, A.H.R. 1909-1934. Researches on Fungi (6 vols.) Longmans, Green and Co., London.
Fell, J. W., Boekhout, T., Fonseca, A. and Sampaio J.P. 2001. Basidiomycetous yeasts. Pp. 1-36. In: The Mycota VII. Systematics and Evolution. Part B. (Mclaughlin, D. J., McLaughlin, E. G. and Lemke, P. A., eds.). Springer-Verlag, Berlin.
Hibbett, D. S., and Binder, M. 2001. Evolution of marine mushrooms. Biol. Bull. 201:319-322.
Hibbett, D. S. and Thorn, R. G. 2001. Homobasidiomycetes. Pp. 121-170. In: The Mycota VII. Systematics and Evolution. Part B. (Mclaughlin, D. J., McLaughlin, E. G. and Lemke, P. A., eds.). Springer-Verlag, Berlin.
Ingold, C.T. 1939. Spore discharge in land plants. Oxford University Press, London, UK.
Ingold, C.T. 1991. A view of the active basidium in heterobasidiomycetes. Mycol. Res. 95: 618-621.
Kirk, P.M., Cannon, P.F., David, J.C., and Stalpers, J. 2001. Ainsworth and Bisby’s Dictionary of the Fungi. 9th ed. CAB International, Wallingford, UK.
Kohlmeyer, J., and Kohlmeyer, E. 1979. Marine Mycology—The Higher Fungi. Academic Press, New York.
McLaughlin, D.J. 1982. Ultrastructure and cytochemistry of basidial and basidiospore development. In: Basidium and Basidiocarp. Wells, K. and Wells, E.K. (eds.) Springer-Verlag, New York.
McLaughlin, D.M., Beckett, A. and Yoon, K.S. 1985. Ultrastructure and evolution of ballistosporic basidiospores. Bot. J. Linnean Soc. 91: 253-271.
McLaughlin, D.J., Frieders, E.M. and L?, Haisheng. 1995. A microscopist's view of heterobasidiomycete phylogeny. Stud. Mycol. 38: 91-109.
Money, N. P. 1998. More g's than the space shuttle: ballistospore discharge. Mycologia 90:547-558.
Mueller, U. G., S. A. Rehner, and T. R. Schultz. 1998. The evolution of agriculture in ants. Science 281: 2034-2038.
Oberwinkler, F. 1977. Das neue System der Basidiomyceten. In: Beitr?ge zur Biologie der niederen Pflanzen. Frey, H., Hurka, H., Oberwinkler, F. (eds.) G. Fischer, Stuttgart.
Oberwinkler, F. 1987. Heterobasidiomycetes with ontogenetic yeast-stages - systematic and phylogenetic aspects. Stud. Mycol. 30: 61-74.
Prillinger, H. D?rfler, C. Laaser, G. and Hauska, G. 1990. A contribution to the systematics and evolution of higher fungi: yeast-types in the basidiomycetes. Part III: Ustilago-type. Z. Mycol. 56: 251-278.
Prillinger, H., Laaser, G., D?rfler, C. and Ziegler, K. 1991. A contribution to the systematics and evolution of higher fungi: yeast-types in the basidiomycetes. Part IV: Dacrymyces-type, Tremella-type. Sydowia 43: 170-218.
Smith, S. E. and Read, D. J. 1997. Mycorrhizal symbiosis. Academic Press, San Diego.
Sugiyama, J., Fukagawa, M, Chiu, S.-W. and Komagata, K. 1985. Cellular carbohydrate composition, DNA base composition, ubiquinone systems, and diazonium blue B color test in the genera Rhodosporidium, Leucosporidium, Rhodotorula and related basidiomycetous yeasts. J. Gen. Appl. Microbiol. 31: 519-550.
Summerbell, R.C. 1985. The staining of filamentous fungi with diazonium blue B. Mycologia 77: 587-593.
Swann, E.C., Frieders, E.M. and McLaughlin, D.J. 1999. Microbotryum, Kriegeria and the changing paradigm in basidiomycete classification. Mycologia 91:51-66.
Swann, E.C., Frieders, E.M. and McLaughlin, D.J. 2001. Urediniomycetes. Pp. 37-56. In: The Mycota VII. Systematics and Evolution. Part B. (Mclaughlin, D. J., McLaughlin, E. G. and Lemke, P. A., eds.). Springer-Verlag, Berlin.
Swann, E.C. and Taylor, J.W. 1993. Higher taxa of basidiomycetes: an 18S rRNA gene perspective. Mycologia 85: 923-936.
Swann, E.C. and Taylor, J.W. 1995. Phylogenetic perspectives on basidiomycete systematics: evidence from the 18S rRNA gene. Canad. J. Bot. 73: S862-S868.
Webster, J., Davey, R.A. and Ingold, C.T. 1984a. Origin of the liquid in Buller's drop. Trans. Br. Mycol. Soc. 83: 524-527.
Webster, J., Davey, R.A., Duller, G.A. and Ingold, C.T. 1984b. Ballistospore discharge in Itersonilia perplexans. Trans. Br. Mycol. Soc. 82: 13-29.
Wells, K., and Bandoni, R. J. 2001. Heterobasidiomycetes. Pp. 85-120. In: The Mycota VII. Systematics and Evolution. Part B. (Mclaughlin, D. J., McLaughlin, E. G. and Lemke, P. A., eds.). Springer-Verlag, Berlin.
Wheeler, Q. and Blackwell, M. 1984. Fungus-insect relationships. Columbia University Press, New York.
Yoon, K.S. and McLaughlin, D.J. 1986. Basidiosporogenesis in Boletus rubinellus II. Late spore development. Mycologia 78: 185-197.
Information on the Internet
- Culture Collection of Basidiomycetes. Institute of Microbiology, Academy of Sciences of the Czech Republic.
- The Microbial World: Basidiomycota.
- Mycological Society of America. Extensive links to sites concerned with Basidiomycota and other fungi.
- Tom Volk’s Fungi. An amazing collection of information, images, and lore about fungi.
- Bioimages: The Virtual Field Guide (UK) Basidiomycota. Collection of Basidiomycota images.
- The WWW Virtual Library: mycology.
- Deep Hypha Research Coordination Network. Deep Hypha is a project to coordinate and provide resources for research in fungal systematics.
- AFTOL: Assembling the Fungal Tree of Life. Collaborative research in fungal phylogenetics.
Title Illustrations
| Scientific Name | Gymnosporangium juniperi-virginianae |
|---|---|
| Comments |
Spore producing structures of the cedar-apple rust fungus (Urediniomycetes) on an apple leaf. This was the "fungus of the month" in May, 1999, at Tom Volk’s Fungi web site |
| Acknowledgements | Photo courtesy D. H. Scott, from APSnet Image of the Week. |
| Body Part | Spore producing structures (Spermogonia-left, and aecia-right) |
| Copyright | © APS Press |
| Scientific Name | Tilletia controversa |
|---|---|
| Comments | Teliospores of the dwarf bunt fungus (Ustilaginomycetes) |
| Acknowledgements | Photo courtesy B. Goates, from APSnet Image of the Week |
| Life Cycle Stage | teliospores |
| Copyright | © APS Press |
| Scientific Name | Amanita rubrovaginata |
|---|---|
| Comments | Fruiting structures of the mushroom Amanita rubrovaginata (Hymenomycetes) |
| Body Part | Fruiting structures |
| Copyright | © 2003 David S. Hibbett |
About This Page
John Taylor, David McLaughlin and Elizabeth Frieders provided helpful comments during the development of an earlier version of this page. Development of this page since 2003 was facilitated by the "Deep Hypha" Research Coordination Network and the "Assembling the Fungal Tree of Life" project (NSF awards DEB-0090301 and DEB-0228657). Thanks to Nicholas P. Money for additional comments.
Department of Plant Biology
220 BioSciences
1445 Gortner Ave.
University of Minnesota
St. Paul, MN 55108
USA
David S. Hibbett
Biology Department
Clark University
950 Main Street
Worcester, MA 01610
USA
Correspondence regarding this page should be directed to David S. Hibbett at
Page copyright © 2003 and David S. Hibbett
- Content changed 18 September 2003
Citing this page:
Swann, Eric and Hibbett, David S. 2003. Basidiomycota. The Club Fungi. Version 18 September 2003 (tol-reviewed). http://tolweb.org/Basidiomycota/20520/2003.09.18 in The Tree of Life Web Project, http://tolweb.org/