Histeridae
Clown beetles
Michael S. Caterino

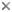
This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
The Histeridae contains about 330 genera and 3900 species (Mazur, 1997). They are mostly predaceous, occurring in a wide variety of habitats for which they are variously specialized. Reputedly typical histerids are shiny, black, ovoid beetles found in dung and carrion. But these represent only a small fraction of true histerid diversity. Many species are associated with dead or dying trees, and other decomposing vegetable matter. Some of the most interesting histerids live in various symbioses, some in vertebrate nests and burrows, and many in the colonies of social insects (Kovarik & Caterino, 2000).
Characteristics
Despite the diversity of body forms in histerids, they may be generally recognized by three main features:
Discussion of Phylogenetic Relationships
The phylogeny of Histeridae has received significant recent attention. The first modern cladistic analysis was performed by Ohara (1994). Although his analysis utilized only 9 informative characters, and a relatively limited taxon set, some interesting results were obtained. In particular, his analysis highlighted the first character evidence that the two longstanding divisions of Histeridae, Saprinomorphae and Histeromorphae (Wenzel, 1944), were likely not monophyletic.
Ohara’s study was expanded upon by Slipinski and Mazur (1999), who analyzed 29 adult characters over a much larger taxon set. Their analysis supported Ohara’s notion that Wenzel’s divisions were artifical, and, significantly, they were the first to provide evidence that the monophyly of some of the subfamilies and tribes was likewise questionable (in particular, Dendrophilinae, Tribalinae, and Exosternini).

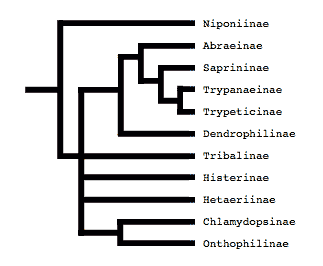
Tree from Slipinski and Mazur (1999)
Both of these studies concluded that Niponius, a cylindrical bark beetle predator from SE Asia, is the most basal extant histerid, and that the shape and habits of this beetle represent the plesiomorphic condition for the family. This concurs with a previous opinion expressed by Roy Crowson (1955), and seems consistent with the accepted hypothesis that the ecomorphologically similar Synteliidae is the sister group of the Histeridae.
The most recent attempt to resolve histerid phylogeny was based on a diverse dataset including adult and larval morphology as well as 18S rDNA sequences (Caterino & Vogler, 2002). That study resulted in the tree presented at the top of this page. Although far from completely resolving histerid phylogeny, this study conflicts seriously with the previous two studies. In particular, the status of Niponius as sister to the remainder of the family finds no support. Rather the most basal groups are suggested to be Onthophilinae and Anapleini. The most significant implication of this resolution is for scenarios of ecomorphological evolution of the family. Instead of having highly specialized subcortical forms as plesiomorphic for the family, it suggests that basal histerids were rather unspecialized, and that various specialized forms and habits have arisen many times during the course of the family’s evolution.
Caterino & Vogler (2002) agree with many of Slipinski & Mazur’s (1999) findings with regard to nonmonophyly of groups like Dendrophilinae, although the specific placements of all of its tribes remain uncertain. Both studies found evidence for a relationship between Bacaniini and Abraeinae. Slipinski and Mazur further placed Anapleini with this group, though Caterino & Vogler’s data found no evidence for this, and instead placed Anapleini at or near the base of Histeridae. Both studies found some evidence of a relationship between the ‘core dendrophilines’, Dendrophilini and Paromalini, although Caterino & Vogler also placed Niponius within this group, possibly as sister group to the latter tribe.
Where Slipinski & Mazur hypothesized the highly specialized cylindrical Trypanaeinae were sister to Abraeinae, Caterino & Vogler suggest that in fact Trypanaeinae are derived from within Abraeinae, and possibly within Teretriini.
Bacaniini have traditionally been included in Dendrophilinae on the basis of a (faintly) prolonged prosternal lobe and exposed antennal cavities, both of which now appear to be plesiomorphies. Slipinski and Mazur (1999) find evidence for a relationship between Bacaniini and Abraeinae in morphological data. Caterino & Vogler (2002) were not able to resolve Bacaniine relationships unambiguously despite the addition of molecular data to morphology.
Histeridae contains two entirely myrmecophilous subfamilies, Hetaeriinae and Chlamydopsinae. Relationships of these have proven particularly difficult to resolve. Most traditional classifications have placed the two as sister families. Recent authors, from Wenzel (1944) onward, have unanimously rejected this view (notwithstanding difficulties finding specific character support for the opinion). Both morphological and molecular data find Hetaeriinae to be closely related to, and probably within, Histerinae. The relationships of Chlamydopsinae, however, remain very obscure. Ohara (1994) suggested a close relationship to Onthophilinae. Slipinski & Mazur’s analysis resulted in a Chlamydopsine/Heteariine sister group relationship, but the authors themselves expressed doubt. Caterino & Vogler’s data were inconclusive, but the authors speculatively agreed with Ohara’s suggestion, with the caveat that Onthophilinae itself is of questionable monophyly.
Onthophilinae and Tribalinae have generally been considered closely related (or have been combined in a single subfamily; Wenzel, 1944; Slipinski and Mazur, 1999.) Ohara (1994) was the first to explicitly disagree with this idea. Analyses presented by Caterino & Vogler (2002) support Ohara's view, resolving Onthophilus and the three examined Tribalinae far apart. However, this cannot be assumed to hold for members of these groups not examined. Convincing synapomorphies for either subfamily remain to be proposed.
Most of the ideas and relationships presented here would benefit greatly from additional examination. 18S proved highly informative for histerid relationships, but many crucial taxa need to be added to this data set. The most desirable additions would include Niponius (Japan & SE Asia), Chlamydopsinae (Indoaustralian), Peploglyptus (a very rare but widespread myrmecophile), Stictostix (Australia & western USA), Sphaericosoma (Madagascar), and Trypolister (South America).
References
Caterino, M. S., and A. P. Vogler. 2002. The phylogeny of the Histeroidea. Cladistics 18(4):394-415.
Crowson, R. A. 1955. The Natural Classification of the Families of Coleoptera. Nathaniel Lloyd, London.
Halstead, D. G. H. 1963. Coleoptera: Histeroidea. Handbooks for the Identification of British Insects 4:1-16.
Kovarik, P. W., and M. S. Caterino. 2001. Histeridae. Pages 212-227 in Arnett, R.H., Jr., and M. C. Thomas. (eds.). American Beetles. Volume 1. Archostemata, Myxophaga, Adephaga, Polyphaga: Staphyliniformia. CRC Press LLC, Boca Raton, FL. xvi + 443 pp.
Kryzhanovskij, O. L., and A. N. Reichardt. 1976. Beetles of the Superfamily Histeroidea. Fauna SSSR, Novaya Seriya 111:1-432.
Mazur, S. 1997. A world catalogue of Histeridae. Genus (Supplement):1-373.
Ohara, M. 1994. A revision of the superfamily Histeroidea of Japan. Insecta Mastumurana (N.S.) 51:1-283.
Slipinski, S. A., and S. Mazur. 1999. Epuraeosoma, a new genus of Histerinae and phylogeny of the family Histeridae. Annales Zoologici (Warszawa) 49:209-230.
Vienna, P. 1980. Coleoptera: Histeridae. Fauna d'Italia 16:1-386.
Wenzel, R. L. 1944. On the classification of histerid beetles. Fieldiana, Zoology 28:51-151 +plates.
Title Illustrations

| Scientific Name | Hololepta |
|---|---|
| Location | Mexico |
| Specimen Condition | Dead Specimen |
| Image Use |
 This media file is licensed under the Creative Commons Attribution-ShareAlike License - Version 3.0. This media file is licensed under the Creative Commons Attribution-ShareAlike License - Version 3.0.
|
| Copyright |
© 2002

|
| Scientific Name | Trypaneus junceus |
|---|---|
| Location | central America |
| Specimen Condition | Dead Specimen |
| Image Use |
 This media file is licensed under the Creative Commons Attribution-ShareAlike License - Version 3.0. This media file is licensed under the Creative Commons Attribution-ShareAlike License - Version 3.0.
|
| Copyright |
© 2002

|
| Scientific Name | Onthophilus giganteus |
|---|---|
| Location | Florida, USA |
| Specimen Condition | Dead Specimen |
| Image Use |
 This media file is licensed under the Creative Commons Attribution-ShareAlike License - Version 3.0. This media file is licensed under the Creative Commons Attribution-ShareAlike License - Version 3.0.
|
| Copyright |
© 2002

|
| Scientific Name | Saprinus discoidalis |
|---|---|
| Location | California, USA |
| Specimen Condition | Dead Specimen |
| Image Use |
 This media file is licensed under the Creative Commons Attribution-ShareAlike License - Version 3.0. This media file is licensed under the Creative Commons Attribution-ShareAlike License - Version 3.0.
|
| Copyright |
© 2002

|
About This Page

Santa Barbara Museum of Natural History, Santa Barbara, California, USA
Page copyright © 2002
All Rights Reserved.
- First online 07 March 2002
Citing this page:
Caterino, Michael S. 2002. Histeridae. Clown beetles. Version 07 March 2002. http://tolweb.org/Histeridae/9223/2002.03.07 in The Tree of Life Web Project, http://tolweb.org/














 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site