Pterotracheoidea
Heteropoda , heteropods, sea elephants
Roger R. Seapy- Atlantidae Rang, 1829
- Carinariidae Blainville 1818
- Pterotracheidae Rafinesque, 1814
Introduction
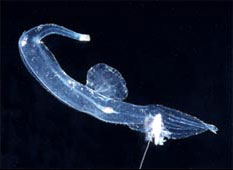
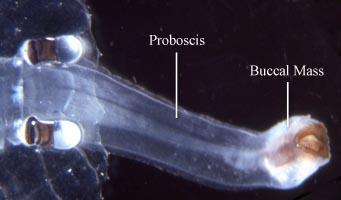
The heteropods are a group of pelagic snails (Class Gastropoda) that are found in moderate to low abundances, primarily in tropical to subtropical latitudes. Among the gastropods, they have four striking adaptations to the open ocean environment. First, the bodies and shells are largely transparent; only the buccal mass, eyes and viscera are opaque. Because of this transparency, internal structures in the body and particularly in the head region are clearly visible (see first photograph below). Second, the foot, which in bottom-dwelling snails is the sole-like structure used for crawling along substrates, is laterally-compressed to form a ventral swimming fin. Third, heteropods swim "upside-down", with the fin and eyes directed upward. Fourth and unique among gastropods, the eyes are image-forming; with a large, spherical lens and a basal, ribbon-like retina (see second photo below). And, lastly, the radula has elongate, sickle-shaped lateral and marginal teeth that are used to snare prey after the radula is protruded from the mouth, which is located at the tip of a mobile proboscis. The presence of a trunk-like proboscis is responsible for their common name, "sea elephants."
Diagnosis
Gastropod mollusks with:- Bodies that are largely transparent
- Laterally-flattened ventral swimming fin
- Paired image-forming eyes with large, spherical lenses
- Radula with elongate, sickle-shaped teeth that are used to snare prey
Characteristics
- Eyes
- Eyes paired, located dorsally in the head region at the anterior end of the trunk. Shape of eyes, viewed dorsally, narrowly to broadly triangular and rectangular
- Eye structure complex, consisting of a distal, large spherical lens, an intermediate region with a pigmented external wall (with or without a clear dorsal window), and a basal ribbon-like retina
- Eye enclosed in gelatinous capsule (visible immediately distal to the lens in the photograph above)
- Retina strip-like; only three (Oxygyrus inflatus) or six (Pterotrachea coronata) photoreceptors in width and several hundred in length (Land, 1982).
- Eye rotation, termed scanning eye movements, enables the brain to create an image. Rotation of the right eye through a 90° arc in Oxygyrus inflatus was captured by M. Vecchione in a video recording: http://nmnh.typepad.com/no_bones/2016/05/the-strange-scanning-eyes-of-a-swimming-sea-snail.html
- Prey are presumably located beneath a predator heteropod by light reflected off the prey's body.

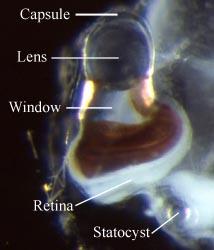
Figure. Left eye of a small (ca. 20 mm body length) Pterotrachea hippocampus in dorsal view. Also in the photograph is the left statocyst, which consists of an outer capsule and an inner, mineralized statolith (at the tip of the pointer). © 2005
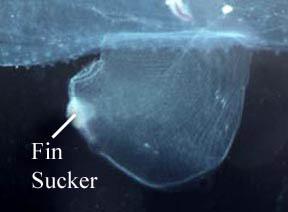
- Swimming fin, sucker and operculum
- Foot laterally compressed as a swimming fin, which usually has a posterior to postero-ventral sucker
- Fin sucker much larger in the Atlantidae than in the Carinariidae and Pterotracheidae (see title illustrations). In the atlantids the sucker serves the function of holding prey while the radula tears off pieces of prey tissues for ingestion. The sucker is much smaller in carinarids and, even more so, in pterotracheids (where it occurs only in males). In both of the latter families the function of the sucker appears to be limited to holding the male and female together during copulation, thus facilitating spermatophore transfer from the male to the female
- Operculum present in all larval stages of heteropods, but is only retained following metamorphosis in the Atlantidae. The operculum is cartilaginous, thin and flexible. It is born on an opercular lobe, located on the posterior margin of the swimming fin. In atlantids the operculum serves to close off the shell aperture after animals retract into their shells
- Proboscis and radula
- Proboscis muscular and mobile, extending anteriorly from the head and bearing a terminal buccal mass that contains the radula
- Radula taenioglossate with a tooth formula of 2-1-R-1-2; i.e., each tooth row consists of two marginal teeth and one lateral tooth on either side of the central or rachidian tooth (R). Lateral and marginal teeth have elongated shafts and curved, hook-shaped ends. Tooth morphology differs between the three families
- Radular tooth shapes change during ontogenesis, with the result that position on the radula of the tooth row can change the appearance of the teeth (Richter, 1961, 1963). Morphogenetic changes in the teeth continue for a large part of radular growth in the Atlantidae, while in the other two families change is restricted to the late larval and early post-larval stages
- Two markedly different types of radulae are distinguished in the heteropods (Richter, 1961). In Type I radulae, the number of tooth rows increases continuously with growth, with the result that all stages of change in tooth shape are represented in mature animals and that teeth produced first on the radula are not cast off the anterior end of the radula after they are no longer of use in prey capture. In Type II radulae, teeth are cast off the anterior end of the radula once the radula has reached its specific number of tooth rows; thus the radula has a limited number of tooth rows. Type II radulae are seen in all Carinariidae and Pterotracheidae, while in the Atlantidae both types are seen (13 species have Type I and 8 species have Type II radulae)
- Shell
- A coiled shell is present in the larvae of all heteropods. Following metamorphosis, however, the larval shell is retained only in the Atlantidae and Carinariidae, where it forms the protoconch of the adult shell. In Pterotracheidae the larval shell is cast off after metamorphosis
- Adult shell in Atlantidae and Carinariidae bears a keel, which extends outward from the last shell whorl in atlantids and anteriorly from the shell in carinariids
 Click on an image to view larger version & data in a new window
Click on an image to view larger version & data in a new window

Figure. Left: Atlanta peronii, viewed from the right side. Note the transparent shell and the keel that extends outward from the shell margin. Right: visceral mass and gills contained within the transparent shell of Carinaria galea, viewed from right side. The well-developed keel extends from the anterior edge of the shell. The stalked visceral mass is attached to the posterior part of the trunk (see the second title illustratoin). © 2005
- In the three genera of the family Atlantidae the shell and keel are either calcareous (Atlanta), composed of a cartilaginous-like material termed conchiolin (Oxygyrus), or with a calcareous shell and a conchiolin keel (Protatlanta). In Carinariidae the adult shell is calcareous.

Figure. Swimming fin with posteroventral sucker in a male Carinaria japonica, viewed from the right side. ©
Comments
The three families comprising the superfamily Pterotracheoidea can be most readily distinguished by the following characters:
| Family | Adult shell | Body can retract into shell | Adult body size | Location of swimming fin |
|---|---|---|---|---|
| Atlantidae | present | yes | microscopic (shell diameter < 1 cm) | extending anteriorly beneath head |
| Carinariidae | present | no | macroscopic (adult body length > ca. 2 cm) | opposite visceral mass at posterior end of trunk |
| Pterotracheidae | absent | not applicable | macroscopic (adult body length > ca. 2 cm) | on trunk ventrum between visceral mass and head |
Life History
Heteropods are dioecious (separate sexes) and exhibit sexual dimorphism. Males have a prominent penis and penial appendage where sperm are packaged into spermatophores (see first photograph below). Sperm are transferred to the penis from the testis, located in the visceral mass, by an external ciliated sperm groove. During copulation, spermatophores are transferred to females. Unfortunately, mating behavior has only been observed in Pterotrachea hippocampus (by R. Harbison; reported in Lalli and Gilmer, 1989). Fertilized eggs are deposited in mucoid egg strings (see second photograph below) that eventually break free from the female, except in Firoloida demarestia which has a permanently attached tubular filament that holds the developing embryos (Owre, 1964; see F. demarestia page).

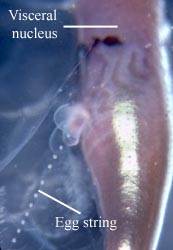
Figure. Left: side view of posterior region of trunk of Carinaria japonica, male. Penis and penial appendage lie parallel to each other; penis contains brown spermatophore. Right: side view of visceral nucleus in Pterotrachea coronata, female. The egg string is released from the oviduct opening, which is surrounded by transparent "lips". © 2005
All heteropods have a free-swimming, planktotrophic veliger larva. Larvae hatch from the egg within a few days of fertilization. They possess a calcareous, dextrally-coiled (right-handed) shell and a flexible, cartilaginous operculum. The larvae have a velum that serves as both a swimming and feeding organ. The velum is initially small and bilobed. With growth each lobe differentiates into two (pterotracheids) or three (atlantids and carinariids) lobes. The number of velar lobes characterize three larval stages termed Stages I, II and III by Thiriot-Quiévreux (1973). Each stage is accompanied by growth changes in the eyes, shell and foot (Thiriot-Quiévreux, 1973; Fig. 5). Following metamorphosis the larval shell is retained as the protoconch of the adult shell in atlantids and carinariids, but is cast off in pterotracheids. The larval operculum is either retained (atlantids) or cast off (carinariids and pterotracheids) at metamorphosis.

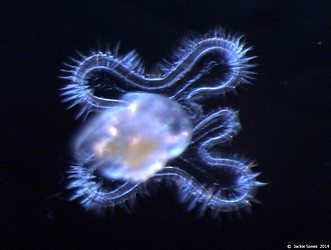
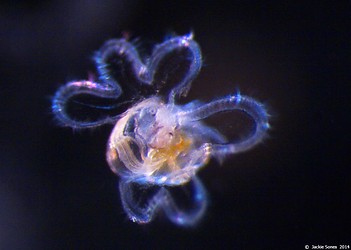
Figure. Top left and right: photographs of stage II and III veliger larvae in Atlanta californiensis. Bottom left: sketch of late stage III larva in Carinaria lamarcki, shown in ventral view (from Thiriot-Quievrèux (1973; Fig. 8). Bottom right: photograph of late stage III larva in C. japonica in dorsal view by Jacqueline Sones (2014). Note the elongate brown patch at the ends of the velar lobes in C. japonica (also reported by Seapy and Thiriot-Quievrèux, 1994), which contrasts with the much shorter brown pigmented area on the lobe ends in C. lamarcki (Thiriot-Quievrèux, 1975).
Natural History
All heteropods swim with their ventral side directed upward and the dorsal side, with the shell (when present) and visceral mass downward. Steady swimming is by undulations of the fin. In atlantids, a considerable side-to-side body motion is induced by the large fin, although the shell and keel act to partially offset the fin's movements. The large, elongate bodies of the carinariids and pterotracheids greatly dampen the side-to-side body motion. In the latter two groups, and especially in the pterotracheids, flexion of the trunk and tail are used in accelerated swimming during pursuit of prey or evasion of predators. The bodies of pterotracheids are the most elongate and streamlined of the three families, and they are the fastest swimmers.
The atlantids are negatively buoyant. During the day they must swim to maintain position. At night, however, they secrete strands of buoyant mucus, up to 0.5 m long, from which they are suspended (Lalli and Gilmer, 1989; Newman, 1990). Carinariids and pterotracheids have been observed to float motionless without sinking (Lalli and Gilmer, 1989). Their neutral buoyancy results from the large amounts of gelatinous tissue, mostly in the trunk and tail, that is made positively-buoyant by ion regulation; i.e., by replacing heavier sulfate ions by lighter chloride ions (up to 75% in Pterotrachea coronata) (Denton and Shaw, 1961).
In all heteropods, prey are captured by elongate, hooked radular teeth (see photographs above). In the atlantids, the fin sucker is large and is used to hold the prey while pieces of tissue are torn off and ingested. In the carinariids and pterotracheids prey are ingested whole. The fin sucker is much smaller in these two groups and does not appear to be used in feeding. It is directed ventrally to posteroventrally and is spatially separated from the mouth; its primary or sole function is to hold males and females together during mating.
Heteropods feed on a variety of zooplanktonic prey. Atlantids feed preferentially on other gastropods, especially shelled pteropods (Richter, 1968; Newman, 1990). Carinarids prefer soft bodied prey (e.g., salps, doliolids and chaetognaths in Carinaria japonica; Seapy, 1980). Little is known of feeding in pterotracheids.
Defense against predators is based largely on transparency, although the buccal mass, pigmented areas of the eyes, and visceral mass (atlantids and carinarids) or visceral nucleus (pterotracheids) are opaque. In Pterotrachea behavioral orientation and surface reflectivity of the pigmented portion of the eyes and visceral nucleus reduce the visibility of these structures to upward-searching predators (Seapy and Young, 1986).
Other Names for Pterotracheoidea
- Heteropoda
- Carinarioidea
- Vernacular Names: heteropods, sea elephants
References
Denton, E. J. and T. I. P. Shaw. 1961. The buoyancy of gelatinous marine animals. Journal of Physiology, London 161: 14P-15P (Proceedings).
Lalli, C. M. and R. W. Gilmer. 1989. Pelagic snails. The biology of holoplanktonic gastropod snails. Stanford: Stanford University Press. 259 pp.
Land, M. F. 1982. Scanning eye movements in a heteropod mollusc. Journal of Experimental Biology 96: 427-430.
Newman, L. 1990. The taxonomy, distribution and biology of Atlanta gaudichaudi Souleyet, 1852 (Gastropoda, Heteropoda) from the Great Barrier Reef. American Malacolological Union Bulletin 8: 85-94.
Newman, L. 1998. Superfamily Carinarioidea, pp. 804-808. In: P. L. Beesley, G. L. B. Ross and A. Wells (eds), Mollusca: the southern synthesis. The Fauna of Australia. Vol. 5, Pt. B. Melbourne: CSIRO Publ.
Owre, H. B. 1964. Observations on development of the heteropod molluscs Pterotrachea hippocampus and Firoloida desmaresti. Bulletin of Marine Science 14: 529-538.
Richter, G. 1961. Die Radula der Atlantiden (Heteropoda, Prosobranchia) und ihre Bedeutung für die Systematik und Evolution der Familie. Zeitschrift für Morphologie und Ökologie der Tiere 50: 163-238.
Richter, G. 1963. Untersuchungen zur Morphogenese der Gastropodenradula. Veröffentlichungen des Instituts für Meeresforschung in Bremerhaven, Drittes meeresbiologisches Symposion 3: 142-152.
Richter, G. 1968. Heteropoden und Heteropodenlarven im Oberflächenplankton des Golfs von Neapel. Pubblicazioni della Stazione Zoologica di Napoli 36: 346-400.
Richter, G. 1974. Die Heteropoden der "Meteor"-Expedition in den Indischen Ozean, 1964/65. "Meteor" Forschungs-Ergebnisse (D) 17: 55-78.
Richter, G. and R. R. Seapy. 1999. Heteropoda, pp. 621-647. In: D. Boltovskoy (ed.), South Atlantic Zooplankton. Backhuys Publishers, Leiden.
Seapy, R. R. 1980. Predation by the epipelagic heteropod mollusk Carinaria cristata forma japonica. Marine Biology 60: 137-146.
Seapy, R. R. and R. E. Young. 1986. Concealment in epipelagic pterotracheid heteropods (Gastropoda) and cranchiid squids (Cephalopoda). Journal of the Zoological Society of London (A) 210: 137-147.
Seapy, R. R., C. M. Lalli and F. E. Wells. 2003. Heteropoda from western Australian waters, pp. 513-546. In: F. E. Wells, D. I. Walker and D. S. Jones (eds.), The Marine Flora and Fauna of Dampier, Western Australia. Western Australia Museum, Perth.
Seapy, R. R. and Thiriot-Quiévreux, C. 1994. Veliger larvae of Carinariidae (Mollusca: Heteropoda) from Hawaiian waters. Veliger 37: 336-343.
Spoel, S. van der. 1976. Pseudothecosomata, Gymnosomata and Heteropoda (Gastropoda). Bohn, Scheltema and Holkema, Utrecht. 484 pp.
Spoel, S. van der, L. Newman and K. W. Estep. 1997. Pelagic molluscs of the world. World Biodiversity Database, CD-ROM Series. Expert Center for Taxonomic Identification (ETI), University of Amsterdam. UNESCO Publishing, Paris.
Tesch, J. J. 1949. Heteropoda. Dana Report 34, 53 pp., 5 plates.
Thiriot-Quiévreux, C. 1973. Heteropoda. Oceanography and Marine Biology Annual Review 11: 237-261.
Thiriot-Quiévreux, C. 1975. Observations sur les larves et les adultes de Carinariidae (Mollusca: Heteropoda) de l'Ocean Atlantique Nord. Marine Biology 32: 379-388.
Title Illustrations

| Scientific Name | Oxygyrus inflatus |
|---|---|
| Location | Hawaiian waters |
| Specimen Condition | Live Specimen |
| Identified By | Roger Seapy |
| Sex | Female |
| Life Cycle Stage | adult |
| View | right side |
| Image Use |
 This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0. This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0.
|
| Copyright |
© 2005

|
| Location | Hawaiian waters |
|---|---|
| Specimen Condition | Live Specimen |
| Sex | Female |
| Life Cycle Stage | adult |
| View | right side |
| Image Use |
 This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0. This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0.
|
| Copyright |
© 2005

|
About This Page

California State University, Fullerton, California, USA
Correspondence regarding this page should be directed to Roger R. Seapy at
Page copyright © 2009
 Page: Tree of Life
Pterotracheoidea . Heteropoda , heteropods, sea elephants.
Authored by
Roger R. Seapy.
The TEXT of this page is licensed under the
Creative Commons Attribution License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Pterotracheoidea . Heteropoda , heteropods, sea elephants.
Authored by
Roger R. Seapy.
The TEXT of this page is licensed under the
Creative Commons Attribution License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
- First online 15 February 2005
- Content changed 09 October 2009
Citing this page:
Seapy, Roger R. 2009. Pterotracheoidea . Heteropoda , heteropods, sea elephants. Version 09 October 2009 (under construction). http://tolweb.org/Pterotracheoidea/27801/2009.10.09 in The Tree of Life Web Project, http://tolweb.org/












 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site