Salamandridae
Newts and "True Salamanders"
Allan Larson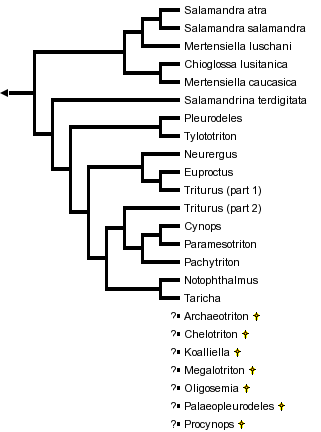

This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxPhylogenetic relationships of extant salamandrid genera are from Titus and Larson (1995). For more information on extinct genera, see Estes (1981) and Duellman and Trueb (1986).
Introduction
Salamanders of the family Salamandridae are informally subdivided into two major subgroups, the "true salamanders" (includes genera Chioglossa, Mertensiella and Salamandra) and the newts (includes all remaining extant genera). Both groups have aquatic larvae except for some viviparous true salamanders (Mertensiella luschani, Salamandra atra and Salamandra salamandra bernardezi) that give birth to fully metamorphosed offspring. Metamorphosed adults of the true salamanders are highly terrestrial whereas those of newts are at least partly aquatic and some are entirely aquatic. Some newts of the North American genus Notophthalmus have a particularly complex life cycle with two metamorphoses and three distinct developmental stages: an aquatic larva, a terrestrial juvenile (the "red eft") and a secondarily aquatic adult.
The family Salamandridae is primarily European and Asian in its distribution. The true salamanders are almost entirely European in distribution, except for some peripheral populations of the widespread and highly variable species, Salamandra salamandra, located in the Middle East (Israel, Lebanon and Syria) and northwest Africa (northern Algeria and Morocco). Habitats favored by the true salamanders include burrows and refuges located under logs or stones in moist woodlands and subalpine meadows. The true salamanders are generally secretive and are active on the surface only on mild, damp nights.
The newts are more widespread in distribution, covering most of Europe and including species located in southeastern China and Vietnam, the Middle East, northwestern Africa and North America. All newts have aquatic larvae and some, such as members of the Chinese genus Pachytriton, are strictly aquatic also as adults. Other newts are largely terrestrial as adults, but all must return to ponds or streams to reproduce.
All salamandrids have toxic skin secretions, and newts are highly poisonous in all stages of their life history. Many salamandrids have bright colors that serve as warnings of their toxicity and may be used in defensive displays.
For a detailed review of the biology, reproduction and diversity of salamandrids, see Griffiths (1996) for European species and Zhao et al. (1988) for Asian species. Halstead (1992) comments on toxicity of newts. Studies of phylogenetic relationships among genera of salamandrids are summarized by Titus and Larson (1995), and their relationships to salamanders of other families are summarized by Larson and Dimmick (1993). For a comprehensive listing of extant species, see Frost (1985) and Duellman (1993), and for fossils, see Estes (1981) and Duellman and Trueb (1986). See also Nussbaum et al. (1995) for recent taxonomic changes in the genus Tylototriton.
Characteristics
Diagnosis
Newts of the family Salamandridae are unlike all other salamanders in having rough-textured skin that is not slimy. Costal grooves usually are not distinct. Most salamandrids have a biphasic life history with aquatic larvae and metamorphosed adults that may be terrestrial or aquatic. The tail is finlike in aquatic forms but not in terrestrial forms. Larvae have four pairs of gill slits and large external gills. Legs are relatively long, prominent and robust.
Detailed Characteristics of the Salamandridae
The morphological characters given below are the ones standardly used to diagnose the salamander family Salamandridae and to assess its phylogenetic relationships to other salamanders. The individual characteristics are in most cases shared with other salamanders and are not synapomorphies of the Salamandridae. Absence of characteristics found in other salamanders is noted where it is important for distinguishing salamandrids from other salamanders and/or determining their relationships to other salamanders. These characteristics were assembled from a large number of original sources by Duellman and Trueb (1986), Larson (1991) and Larson and Dimmick (1993). See "Discussion of Character Evolution" below for comments on some interesting evolutionary trends occurring within the Salamandridae.
Morphology of the skull
The premaxilla may consist of a single bone or separated, paired bones. The angular bone is fused to the mandible. A frontosquamosal arch is present in some genera. Bilaterally paired nasal bones each ossify from a single, laterally positioned anlage; long posterior processes of the premaxillae extend between the paired nasal bones and completely separate them. Septomaxillary bones are absent. Lacrimal bone is absent. Quadratojugal bone is absent. Pterygoid bones are present. Internal carotid foramina are absent from parasphenoid bones. Ear bones feature fusion of the columella with the ear capsule, and a free operculum. Replacement of vomerine teeth proceeds medially. Teeth have a distinct crown and pedicel. Origin of the levator mandibulae anterior superficialis muscle includes the exoccipital.
Inner ear
A basilaris complex is present in the inner ear of some species and absent from others. The recessus amphibiorum is oriented horizontally in the inner ear. The otic sac is multilobate, vascularized and filled with calcium. Fibrous connective tissue is present around the amphibian periotic canal. The periotic cistern is small. The periotic cistern may or may not protrude into the fenestra.
Hyobranchial structures
The first hypobranchial and first ceratobranchial (alternatively homologized as the first ceratobranchial and first epibranchial, respectively) exist as separate structures. The second ceratobranchial (alternatively homologized as the second epibranchial) comprises a single element. Lungs are present but greatly reduced in some species. An ypsiloid cartilage is present. Larvae have four pairs of gill slits and large external gills.
Postcranial Morphology
The scapula and coracoid bones of the pectoral girdle are fused to form the scapulocoracoid. Vertebral centra are opisthocoelous. Ribs are bicapitate. Spinal-nerve foramina are present in neural arches of all vertebrae except for spinal nerves exiting between the atlas and first trunk vertebra. The pubotibialis and puboischiotibialis exist as separate muscles. Anterior glomeruli of the kidney are reduced or absent.
Reproductive characters
Fertilization is internal. Ciliated epithelium may be present or absent in the cloacal tube and anterior cloacal chamber of females. Epidermal lining is absent from the anterior cloacal chamber of females. Evaginations are absent from the dorsolateral walls of the male cloacal tube. Anterior ventral glands are present in the cloacae of females. Spermathecae are present in the female cloacal chamber. Glands secreting into the dorsal walls of the female cloaca are absent. Anterior ventral glands are present in male cloacae. Posterior ventral glands are present in male cloacae. Kingsbury's glands are present in male cloacae. Dorsal pelvic glands are present in males. Lateral pelvic glands are absent in males. Glands secreting into the male cloacal orifice are present. Parental care of eggs has not been reported.
The diploid number of chromosomes is 22 (Notophthalmus and Taricha) or 24 (see Morescalchi, 1975).
Classification
Although taxonomists generally have accepted the hypothesis of monophyly for extant salamandrid genera, evidence for salamandrid monophyly rests mainly on recent molecular data (Larson, 1991; Titus and Larson, 1995).
The "true salamanders" and "newts" are not recognized as formal taxa, although the results of Titus and Larson (1995) are compatible with the interpretation that these groupings represent monophyletic sister taxa and potentially could be recognized as subfamilies. The exact phylogenetic position of the newt, Salamandrina terdigitata, is uncertain (see below) and requires better resolution before we can be confident that the newts form a monophyletic group.
The results of Titus and Larson (1995) suggest that the genera Mertensiella and Triturus, as currently recognized, are not monophyletic groups. Further work is needed to resolve in detail the phylogenetic relationships of species placed in these genera, but future taxonomic revisions are likely for both genera.
Discussion of Phylogenetic Relationships
Titus and Larson (1995) review 48 morphological and reproductive characteristics that contain phylogenetic information on relationships among genera of the Salamandridae (see also Özeti and Wake, 1969; Wake and Özeti, 1969; Sever, 1992). No single feature uniquely characterizes all salamandrids as a monophyletic group, but a combined analysis of these characters and variation in ~1,000 bases of mitochondrial DNA sequence strongly supports monophyly of the Salamandridae. This analysis of the combined morphological and molecular characters gives strong support to the monophyly of the true salamanders (genera Chioglossa, Mertensiella and Salamandra) and to monophyly of a group containing all newts except Salamandrina terdigitata.
The genus Salamandrina appears to be the sole extant representative of an ancient lineage that separated from other salamandrid lineages near the time that the lineages leading to the remaining newts and to the true salamanders were formed. Salamandrina is tentatively shown as being closer to the other newts than to the true salamanders, but further work conceivably could reveal that it is closer to the true salamanders. Salamandrina is clearly only a distant relative of the other salamandrid genera, and we can be fairly confident that it does not belong within either the clade containing the remaining newts or the clade containing the true salamanders.
Within the true salamanders, Salamandra is clearly a monophyletic genus and a group containing Salamandra plus Mertensiella luschani appears monophyletic (Titus and Larson, 1995). Mertensiella caucasica appears closer phylogenetically to Chioglossa than to M. luschani and Salamandra, which makes Mertensiella nonmonophyletic. The main morphological characteristic used to diagnose Mertensiella is presence in males of a cutaneous dorsal papilla near the base of the tail. This papilla may function in the mating ritual but its exact role is unclear. This characteristic might have been present in the most recent common ancestor of all true salamanders and subsequently lost in the lineages leading to Chioglossa and Salamandra, which would make Mertensiella paraphyletic; alternatively, the papilla might have evolved in parallel in the lineages leading to the two species of Mertensiella, which would make the genus polyphyletic.
Within the newts, phylogenetic evidence gives strong support to monophyly of the genus Tylototriton and to a grouping of Tylototriton with Pleurodeles (Titus and Larson, 1995). Tylototriton and Pleurodeles form the sister taxon to a monophyletic group containing all remaining newts except Salamandrina. Within the latter clade, monophyly is well-supported for a group containing three Asian genera (Cynops, Pachytriton and Paramesotriton) and a group containing the European genera Euproctus, Neurergus and part of Triturus (Titus and Larson, 1995). Monophyly of a group containing the North American genera, Notophthalmus and Taricha, receives weak support from the analysis of character variation (Titus and Larson, 1995). All of the remaining groupings shown on the tree are more tentative.
The large European genus Triturus appears not to be monophyletic, with at least one species being closer phylogenetically to Euproctus than to other species of Triturus. Further analyses incorporating all species of Triturus plus the other newt genera are needed to resolve the phylogenetic relationships of the Triturus species to the other newts.
Discussion of Character Evolution
Knowledge of phylogenetic relationships permits interpretation of the evolution of several morphological characters that have been important in the evolutionary diversification of salamandrids. Rough, keratinized skin characterizes all newts except the highly aquatic species, Pachytriton labiatum. Rough skin appears to have evolved in a common ancestor of the newts after their separation from the true salamanders, followed by loss of keratinized skin in the Pachytriton lineage.
Evolution of courtship in salamandrids appears to involve separate evolutionary origins of mating behaviors in which the male actively captures the female on its ventral surface (Mertensiella, Salamandra, Pleurodeles, and Tylototriton), dorsal surface (Notophthalmus and Taricha) or tail (Euproctus). Capture of the female by the male is absent in the other genera, and absence of capture is considered the ancestral condition for salamandrids. Additional data on the courtship of Salamandrina, which currently is unknown, are required to clarify the evolutionary sequence of changes in courtship behavior.
The phylogenetic analysis suggests that egg-laying is ancestral for salamandrids and that the live-bearing observed in Mertensiella and Salamandra is evolutionarily derived. Presence of well-developed lungs is ancestral for salamandrids with three evolutionarily independent reductions or losses of lungs in the genera Chioglossa, Euproctus and Salamandrina.
Two cranial characters provide synapomorphies for subgroups of salamandrids (Titus and Larson, 1995). Presence of a frontosquamosal arch is a derived characteristic found in all newts but no other salamanders. Fusion of the premaxillary bones in salamandrids is a synapomorphy of all newts excluding the genera Pleurodeles, Salamandrina and Tylototriton, although this character has arisen independently in other salamanders (see Wake and Larson, 1987). Other cranial characteristics show more complex patterns of evolution in the Salamandridae.
References
Duellman, W. E. 1993. Amphibian Species of the World: Additions and Corrections. Univ. of Kansas Printing Service. Lawrence, Kansas.
Duellman, W. E. and L. Trueb. 1986. Biology of Amphibians. McGraw-Hill, New York.
Estes, R. 1981. Gymnophiona, Caudata. Handbuch der Paläoherpetologie 2:1-115.
Frost, D. R. 1985. Amphibian Species of the World. Allen Press and the Association of Systematics Collections. Lawrence, KS.
Griffiths, R. 1996. Newts and Salamanders of Europe. T & AD Poyser Ltd., London.
Halstead, B. W. 1992. Dangerous Aquatic Animals of the World. The Darwin Press Inc. Princeton, New Jersey (published simultaneously by Mosby-Year Book, Inc. St. Louis, Missouri)
Larson, A. 1991. A molecular perspective on the evolutionary relationships of the salamander families. Evolutionary Biology 25:211-277.
Larson, A. and W. W. Dimmick. 1993. Phylogenetic relationships of the salamander families: A analysis of congruence among morphological and molecular characters. Herpetological Monographs 7:77-93.
Morescalchi, A. 1975. Chromosome evolution in the caudate Amphibia. Evolutionary Biology 8:339-387.
Nussbaum, R. A., E. D. Brodie, Jr. and Y. Datong. 1995. A taxonomic review of Tylototriton verrucosus Anderson (Amphibia: Caudata: Salamandridae). Herpetologica 51:257-268.
Özeti, N. and D. B. Wake. 1969. The morphology and evolution of the tongue and associated structures in salamanders and newts. Copeia 1969:91-123.
Sever, D. M. 1992. Comparative anatomy and phylogeny of the cloacae of salamanders (Amphibia: Caudata). IV. Salamandridae. Anatomical Record 233:229-244.
Titus, T. A. and A. Larson. 1995. A molecular phylogenetic perspective on the evolutionary radiation of the salamander family Salamandridae. Systematic Biology 44:125-151.
Wake, D. B. and A. Larson. 1987. Multidimensional analysis of an evolving lineage. Science 238:42-48.
Wake, D. B. and N. Özeti. 1969. Evolutionary relationships in the family Salamandridae. Copeia 1969:124-137.
Zhao, E., Y. Jiang, Q. Hu and Y. Yang. 1988. Studies on Chinese Salamanders. Society for the Study of Amphibians and Reptiles. Oxford, Ohio.