Holothuroidea
Sea cucumbers
Alexander M. Kerr

This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxTree modified from Kerr (2000).
Introduction
The Holothuroidea, or sea cucumbers, are an abundant and diverse group of worm-like and usually soft-bodied echinoderms. They are found in nearly every marine environment, but are most diverse on tropical shallow-water coral reefs. They range from the intertidal, where they may be exposed briefly at low tide, to the floor of the deepest oceanic trenches. The oldest undoubted fossils of sea cucumbers are of isolated spicules from the Silurian (ca. 400 million years ago; Gilliland, 1993). Considerable diversification has occurred since then with about 1400 living species in a variety of forms. Some of these are about 20 cm in length, though adults of some diminutive species may not exceed a centimeter, while one large species can reach lengths of 5 m (Synapta maculata). Several species can swim and there are even forms that live their entire lives as plankton, floating with the ocean currents.
Economically, sea cucumbers are important in two main ways. First, some species produce toxins that are of interest to pharmaceutical firms seeking to learn their medical value. Some compounds isolated to date exhibit antimicrobial activity or act as anti-inflammatory agents and anticoagulants. Second, as a gourmet food item in the orient, they form the basis of a multimillion-dollar industry that processes the body wall for sale as beche-de-mer or trepang. However, the high value of some species, the ease with which such shallow-water forms can be collected and their top-heavy age structures all contribute to over-exploitation and collapse of the fisheries in some regions. Fishermen in the Pacific islands use the toxins, some of which act as respiratory inhibitors, to entice fish and octopus from crevices so that they may be more easily speared. Furthermore, the sticky Cuvierian tubules (see description below) are placed over bleeding wounds as a bandage.
Characteristics
The most important feature distinguishing the sea cucumbers is a calcareous ring that encircles the pharynx or throat. This ring serves as an attachment point for muscles operating the oral tentacles and for the anterior ends of other muscles that contract the body longitudinally. Sea cucumbers are also distinct as echinoderms in having a circlet of oral tentacles. These may be simple, digitate (with finger-like projections), pinnate (feather-like), or peltate (flattened and shield-like). A third key feature, found in 90% of living species, is the reduction of the skeleton to microscopic ossicles (Figure 1). In some species, the ossicles may be enlarged and plate-like.
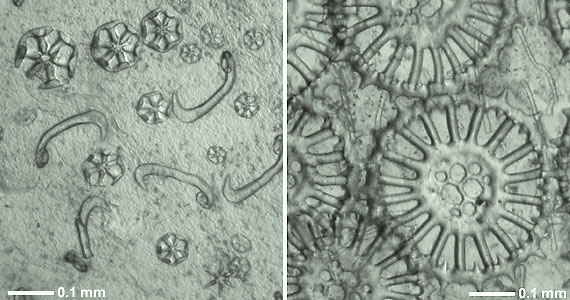 image info
image info
Figure 1. Calcareous skeletal ossicles from the body wall (in situ) of two recent holothurians.
Left: Wheels and hook-shaped rods of Trochodota allani (Apodida: Chiridotidae).
Right: Spinose wheels with perforated hub and simple rods of Siniotrochus phoxus (Apodida: Myriotrochidae).
Photographs copyright © 2000 Mike Reich.
As in other echinoderms, the holothurian water vascular system consists of an anterior ring canal from which arise long canals running posteriorly (not shown in Figure 2). Despite their similarity to the radial canals of other echinoderms, these latter structures arise embryologically in a quite different manner. For this reason these canals in holothurians have been recently renamed longitudinal canals (Mooi and David 1997). In holothurians, the larval structures that would form the radial canals in other echinoderms instead become the five primary tentacles. Also, holothurians with the exception of members in Elasipodida have a madrepore that opens into the coelom (body cavity). In contrast, elasipodans and nearly all other echinoderms have a madrepore that opens externally.
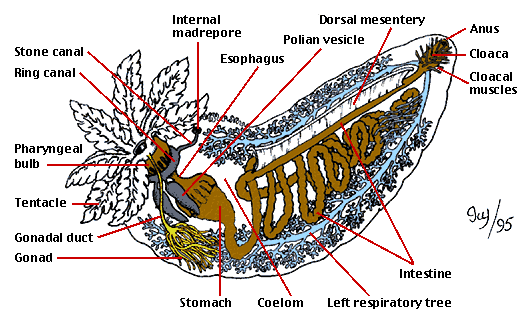 image info
image info
Figure 2: Main internal anatomical features of a cucumariid sea cucumber (Dendrochirotida).
Drawing by Ivy Livingstone. Copyright © 1995 BIODIDAC.
Some sea cucumbers possess organs not found in other invertebrates. In some Aspidochirotida, the respiratory trees display Cuvierian tubules. In most species, these are apparently defensive structures. They can be expelled through the anus, whereupon they dramatically expand in length and become sticky, entangling or deterring would-be predators, such as crabs and gastropods. Many forms, with the exception of members of Elasipodida and Apodida, possess respiratory trees used in gas exchange. These are paired, heavily branched tubes attached to the intestine near the anus. This type of breathing ("cloacal breathing") is also present in an unrelated group, the echiuran worms.
Hyman (1955) provides a useful account of holothuroid gross anatomy, Smiley (1994) covers microscopic aspects, while Smiley et al. (1991) reviews reproduction and larval development.
The Orders of Holothuroidea
The ancestors of the Apodida, Elasipodida and the lineage leading to the remaining orders diverged in the middle to late Paleozoic Era between about 350 to 250 million years ago. The Aspidochirotida, Molpadiida, Dendrochirotida and Dactylochirotida began diverging somewhat later in the Triassic and Jurassic of the early Mesozoic Era about 200 million years ago. Assignment to different orders is largely based on the form of the calcareous ring and tentacles, as well as the presence of certain organs, such as the respiratory trees or the muscles that retract the oral region.
Descriptions of each order given below are modified from Pawson (1982) and Smiley (1994):
- Apodida
- Contains about 269 species in 32 genera and three families. Tentacles are digitate, pinnate or, in some small species, simple. Respiratory trees are absent. Tube feet are completely absent. The calcareous ring is without posterior projections. The body wall is very thin and often transparent. Found in both shallow and deep water.
- Elasipodida
- Contains about 141 species in 24 genera and five families. Tentacles are shield-shaped and used in shovelling sediment. Respiratory trees are present. The calcareous ring is without posterior projections. With the exception of Deimatidae, the body wall is soft to gelatinous. All forms live in deep water.
- Aspidochirotida
- There are about 340 species in 35 genera and three families. Tentacles are shield-shaped. Respiratory trees are present. The calcareous ring is without posterior projections. The body wall is generally soft and pliant. Most forms live in shallow water, though one family is restricted to the deep sea.
- Molpadiida
- There are about 95 species in 11 genera and four families. Tentacles are simple. Respiratory trees are present. The calcareous ring is without posterior projections. The body wall is generally soft and pliant. Most forms live in shallow water, though one family is restricted to the deep sea.
- Dendrochirotida
- Contains about 550 species in 90 genera and seven families. Tentacles are highly branched and extended to filter material from the water column. Respiratory trees are present. Some members with a calcareous ring composed of numerous small pieces or having long posterior extensions. Possess muscles for retracting the oral introvert. The body wall may be hardened from enlarged plate-like ossicles. Live either attached to hard bottoms or burrow in soft sediment. Most species live in shallow water.
- Dactylochirotida
- Contains about 35 species in seven genera and three families. Tentacles are simple or with a few small digits. Respiratory trees are present. The calcareous ring is without posterior projections. Possess muscles for retracting the oral introvert. All members have a rigid body encased in enlarged flattened ossicles. The body is usually "U" shaped. All members live burrowed in soft sediment. Most live in deep water.
Discussion of Phylogenetic Relationships
The evolutionary relationships of the major holothuroid lineages were, until quite recently, poorly understood. This was in part due to their lack of an integrated skeleton like that providing the extensive fossil record and numerous morphological characters of other groups of echinoderms. There have been numerous speculations about the relationships within Holothuroidea extending well back into the 19th century. The methods of modern comparative biology had not been applied to these problems until quite recently. Then Littlewood et al. (1997), in an effort to resolve class-level relationships within echinoderms, sequenced two ribosomal genes from a total of four orders. Their analyses consistently supported a close relationship between Dendrochirotida and Aspidochirotida, but they could not resolve the phylogenetic position of Elasipodida and Apodida (Figure 3: A, B). Smith (1997) subsequently argued that the Elasipodida are more closely related to (Dendrochirotida + Aspidochirotida) than the Apodida (Figure 3: C). This hypothesis recalls an early speculation (Semper 1868) whereby Apodida is sister to the remaining holothuroids.
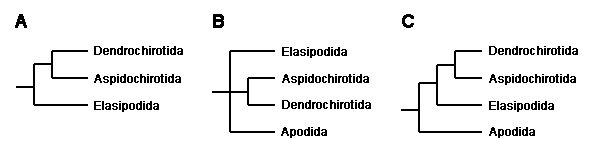 image info
image info
Figure 3. Recent hypotheses about holothuroid relationships.
A. Tree based on complete 18S rDNA sequences (Littlewood et al., 1997).
B. Tree based on partial 28S rDNA sequences (Littlewood et al., 1997).
C. Interpretation of the 18S and 28S rDNA data favored by Smith (1997).
More comprehensive cladistic analyses of morphological and DNA data (Kerr, 2000) agree with Smith's hypothesis. Further, it appears that Dendrochirotida is paraphyletic due to the Dactylochirotida lineage arising from within the Dendrochirotida. This arrangement of the two orders is so far supported by few characters, and an alternate arrangement may ultimately prevail. Kerr (2000) also places Molpadiida as sister to Dendrochirotida plus Dactylochirotida. Together with Aspidochirotida, the aforementioned orders form a group united, most notably, by the presence of respiratory trees. The placement of two rare families currently referred to the Molpadiida, Eupyrgidae and Gephyrothuriidae, is uncertain; they may turn out to be only distantly related to one another and other ordinal level groups of holothurians. Based on the presence of respiratory trees, however, they are unlikely to be closely related to either the Apodida or Elasipodida, which lack such structures. The remaining features of the higher level relationships shown in the figure at the top of this page, though, appear solidly supported and unlikely to change with the consideration of new characters.
Fossil History
As for most soft-bodied animals, holothuroids have a poor fossil record. Published accounts exist of body fossils for about 19 species, though at least that many body-fossil species lay undescribed on museum shelves. Most ancient holothuroids are known from fossils of isolated ossicles. This complicates the taxonomy somewhat since ossicles can differ even within an individual depending on age, habitat and geography. How then does one identify a single species? As a result, most fossil holothuroids have been described as paraspecies based on unique ossicle types. Entire or isolated elements of the calcareous ring are also known, though less work has been done on these potentially informative structures. The rarity of holothuroid fossils in part may be due to a lack of collecting effort, since the microscopic ossicles require special collecting methods, and there are few specialists working on the group. In addition, isolated ring elements may sometimes be confused with the robust plates of other echinoderms.
 image info
image info
Figure 4. Isolated pieces of the calcareous rings of fossil holothurians.
Left: Interradial pieces; Center: Radial pieces; both from apodid holothurians from the Upper Liassic of Germany, approx. 180 Ma;
Right: Pieces from fossil molpadiid holothurians from the Hauterivian of Germany, approx. 130 Ma.
Photographs copyright © 2000 Mike Reich.
Holothuroids probably evolved by at least the Lower Silurian, most likely from a little-known group of extinct Palaeozoic echinoderms called ophiocistioids. However, the oldest reported body fossil of a holothuroid is from the Lower Devonian, while the oldest undoubted ossicle is from the Upper Silurian. Plate ossicles are known from the Ordovician, but their identity as holothuroid is uncertain because they resemble the plates of other echinoderms. Still, plate ossicles ascribable to holothuroids are well known and, when combined with the phylogenetic evidence, suggest that several groups of ancient plated forms existed that are only distantly related to living plated dendrochirotes and dactylochirotes. Alternatively, these living forms have retained their ancient armour and Holothuroidea has had a long and repeated history of losing a plated morphology.
A comprehensive account of holothurian palaeontology is found in Gilliland (1993), while an up-to-date bibliography and other palaeontological information is available from Mike Reich's Fossil Holothuroidea Page.
References
Gilliland, P. M. 1993. The skeletal morphology, systematics and evolutionary history of holothurians. Special Papers in Palaeontology 47: 1-147
Hyman, L. H. 1955. The Invertebrates. Vol. 4. Echinodermata. New York: McGraw Hill.
Kerr, A. 2000. Evolution and Systematics of Holothuroidea (Echinodermata). Thesis, Yale University.
Littlewood, D. T. J., A. B. Smith, K. A. Clough and R. H. Emson. 1997. The interrelationships of the echinoderm classes: morphological and molecular evidence. Biological Journal of the Linnean Society 61: 409-438.
Mooi, R. and B. David. 1997. Skeletal homologies of echinoderms. Paleontological Society Papers 3: 305-355.
Pawson, D. L. 1982. Holothuroidea. In: Parker, S. P., ed. Synopsis and Classification of Living Organisms. New York: McGraw-Hill, 813-818.
Semper, C. 1868. Reisen im Archipel der Philippinen. 2. Wissenschaftliche Resultate. 1. Holothurien. Leipzig: Wilhelm Engelmann.
Smiley, S., F. S. McEuen, S. Chaffee, and S. Krishnan. 1991. Echinodermata: Holothuroidea. In: Giese, A. C., J. S. Pearse, and V. B. Pearse, eds. Reproduction of Marine Invertebrates. Volume 6. Pacific Grove, California: Boxwood Press, 663-750.
Smiley, S. 1994. Holothuroidea. In: Harrison, F. W. and F.-S. Chia, eds. Microscopic Anatomy of Invertebrates. Volume 14. Echinodermata. New York: Wiley-Liss, 401-471.
Smith, A. B. 1997. Echinoderm larvae and phylogeny. Annual Review of Ecology and Systematics 28: 219-241.
Information on the Internet
- UCMP Berkeley Holothuroidea.
- Beche-de-mer Information Bulletin. Coordinated by Chantal Conand.
- Taxonomy of Sea Cucumbers. Philip Lambert, Royal British Columbia Museum.
Title Illustrations
| Scientific Name | Synaptula (Apodida) |
|---|---|
| Location | coral reef on the Eastern Coast of Thailand |
| Specimen Condition | Live Specimen |
| Copyright | © 2000 Sumaitt Putchakarn |
| Scientific Name | Holothuria (Halodeima) atra (Aspidochirotida) |
|---|---|
| Location | coral reef on the Eastern Coast of Thailand |
| Specimen Condition | Live Specimen |
| Copyright | © 2000 Sumaitt Putchakarn |
| Scientific Name | Cucumaria (Dendrochirotida) |
|---|---|
| Location | Ross Sea, Antarctica |
| Specimen Condition | Live Specimen |
| Copyright | © 2000 Norbert Wu |