Salientia
Frogs and toads
David Cannatella, Linda Ford, and Lori Bockstanz

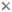
This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
Salientia (Anura) includes frogs, toads and their close fossil relatives. The closest living relatives of the Salientia are the other amphibians, salamanders and caecilians. Frogs outnumber both of these groups substantially; there are 162 living species of caecilians, 352 living salamanders, and 3438 species of frogs (Duellman and Trueb, 1986).
The earliest known salientian is †Triadobatrachus massinoti, from the Early Triassic of Madagascar. This "proto-frog" is about 250 million years old. We call it that because it had not yet quite evolved the combination of features that we now think of as being associated with frogs. For more information see Triadobatrachus massinoti.
The earliest "true" frog is †Vieraella herbsti, from the Early Jurassic era (188-213 my). Thus, perfectly respectable frogs were around just before most of the major groups of dinosaurs had appeared. †Notobatrachus degiustoi from the Middle Jurassic is just a bit younger, about 155-170 million years old.
There are many distinctive features of living frogs. Frogs have at most nine vertebrae in front of the sacrum, and the three or four posterior to the sacrum are fused into a rod called the urostyle. In contrast, caecilians and salamanders have many more vertebrae and they do not have a urostyle. Frogs do not possess tails in the adult stage ("Anura" means without tail), as caecilians and salamanders do. Frogs also have a radioulna, which represents a fused radius and ulna (bones of the forearm), and a tibiofibula, the fused tibia and fibula (bones of the shank). The tibiale and fibulare (ankle bones; also called astragalus and calcaneum) of frogs are greatly elongate. Thus there is effectively an additional lever system that frogs can utilize in jumping. Indeed, the origin of saltation and its morphological correlates (lack of a tail, reduction in vertebrae, elongation of propulsive segments of the body) seems to be one of the features that clearly sets frogs apart from other major vertebrate groups (Gans and Parsons, 1966).
In addition to morphological distinctions, frogs also have a distinctive life phase known as the tadpole, which is a highly specialized "eating machine." Salamanders and caecilians have a larval form, but in neither does the larva possess the many specializations (such as the ceratohyal pump) that frog tadpoles have (Wassersug, 1974). Even the most basal living frogs have the beginnings of a unique mechanism of tongue projection (Nishikawa and Cannatella, 1991; Nishikawa and Roth, 1991) that is associated with extreme modification of the gill arches into a fused hyobranchial plate.
Although there is no scientific distinction between "frogs" and "toads", frogs are typically smooth-skinned, have long hind limbs for leaping, and live in water, while toads have warty, drier skin, with shorter hind limbs for hopping, and live on land (Halliday and Adler, 1986).
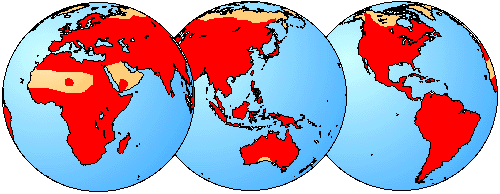
With so many species of frogs and toads, it is not surprising that they inhabit a wide variety of habitats. Habitat types range from arid desert regions to mountainous regions to swamps to tropical rainforests.
Temperature and water regulation are critical to frogs and toads, and amphibians in general. Being ectotherms, frogs and toads are reliant on the ambient temperature for body temperature regulation. In the winter months, frogs in temperate zones cannot remain active and must enter into a state of torpor, or extremely reduced activity. In the contrasting summer months, frogs can avoid the extreme heat by remaining underground in daylight, and being active at night (Halliday and Adler, 1986).
Salientians are also susceptible to the loss of body water due to extremely hot or dry conditions. Moisture regulation in frogs varies with their habitat. Those in temperate climates maintain moist skin to aid in evaporative cooling. As external air passes over the moist skin, the frog's body temperature is lowered. Additionally, permeable skin allows the frog the ability to absorb water simply by jumping into a pond or sitting in a puddle. Frogs in arid regions, on the other hand, have different ways of regulating body water. Their skin is often impermeable to water to prevent rapid evaporation and dehydration. Instead, they may cover their bodies with a thick mucus, or burrow to avoid the heat altogether.
Breeding in frogs is triggered by environmental cues such as temperature change and rainfall. During the breeding season (which varies with each species) hundreds or thousands of frogs may be seen in a congregation. Male frogs attract mates by calling; often many males call in chorus. Calling usually occurs near a body of water, such as a pond, where the eggs can be laid and fertilized. Egg masses may be laid in long chains or in large clumps. Parental care in frogs and toads is variable; some species lay many smaller eggs and have little parental care, while others lay a few larger eggs and remain with them until tadpoles or froglets develop.
Herpetological Fauna of Texas
The Salientia clade includes all frogs and toads from around the world. For a list of only the frog families and species found in Texas, as well as those for other amphibians and reptiles, click on the Herps of Texas icon below. (Under construction)
 Herps of Texas
Herps of Texas
Discussion of Phylogenetic Relationships
Salientia
The name Salientia generally has referred to †Triadobatrachus + Anura (Milner, 1988). Ford and Cannatella (1993) defined it as a stem-based name for amphibians that are more closely related to Anura than to Caudata or Gymnophiona. Synapomorphies that unite all of the currently known taxa in Salientia include 14 presacral vertebrae, elongate and anteriorly directed ilium, presence of a frontoparietal, and a toothless dentary (Milner, 1988). To these, Trueb and Cloutier (1991) added the absence of a lacrimal and unicapitate ribs as other unique synapomorphies, and four other synapomorphies that showed homoplasy among closely related dissorophoid temnospondyls.
Anura (Node A)
The node-based name Anura was defined by Ford and Cannatella (1993) as the last common ancestor of living frogs and all its descendants. According to this definition, †Triadobatrachus is not part of Anura, following Trueb and Cloutier (1991). The late Jurassic fossil †Notobatrachus degiustoi may or may not be part of Anura, depending on whether it is the sister-group of Anura (Cannatella, 1985) or possibly more closely related to Leiopelma (Estes and Reig, 1973). †Vieraella herbstii is another relatively complete early Jurassic fossil, but is less well-preserved than †Notobatrachus (Estes and Reig, 1973). The presence of nine presacral vertebrae places it among the basal salientians, but other characters are not sufficiently preserved to permit definitive placement in Anura. The third well-preserved Jurassic fossil taxon, †Eodiscoglossus santonje, has eight presacral vertebrae (Estes and Reig, 1973), and thus is clearly within Anura. The other Jurassic frogs are not sufficiently diagnosable to be relevant to a discussion of the content of the Anura.
Synapomorphies of Anura include nine presacral vertebrae, atlas with a single centrum, presence of a urostyle formed from caudal vertebral segments, hindlimb longer than forelimb, fusion of radius and ulna, fusion of tibia and fibula, elongate ankle bones (tibiale and fibulare = astragalus and calcaneum), absence of a prefrontal, fusion of hyobranchial elements into a hyoid plate, presence of keratinous beaks and denticles on larval mouthparts, a single median spiracle in the larva (a characteristic of the Type 3 tadpole), skin with large subcutaneous lymph spaces, and two protractor lentis muscles attached to lens (Milner, 1988, 1993; Saint-Aubain, 1981; Trueb and Cloutier, 1991).
The monophyly of Anura has rarely been questioned. Griffiths (1963:279) considered Anura to be diphyletic; i.e., Ascaphus and Leiopelma comprised one lineage, and all other frogs a second, "...stemming independently from either different levels of a single proanuran organization or different proanuran stocks." Rocek (1981) considered †Triadobatrachus to be within Anura, and placed Pelobatidae (including only Pelobates and some related fossils), and somewhat tentatively, Pipidae, †Palaeobatrachidae, and Rhinophrynidae, in Archaeosalientia. †Triadobatrachus and remaining anurans were included in Neosalientia. The character distinguishing the two groups is a median dermal bone, the interparietal (Reinbach, 1939), which Rocek homologized with the median extrascapular of the osteolepiform fish †Eusthenopteron. The interparietal is found in Pelobates and some fossil relatives, but not in any pipoid frogs. Milner (1988) provided a cogent review of the interpretation of this dermal element.
In a study of tetrapod phylogeny, Hedges et al. (1990) analyzed 123 phylogenetically informative sites from 18S ribosomal RNA of 21 tetrapods, including four salamanders, four caecilians, and one species each from Bufonidae, Discoglossidae, Hylidae, Leptodactylidae, Microhylidae, Pelobatidae, and Sooglossidae. Bootstrap analyses using both maximum-parsimony and neighbor-joining algorithms did not support the monophyly of Anura, Caudata, or Gymnophiona, but a monophyletic Amphibia was supported with a bootstrap value of 100%. Hedges and Maxson (1993) and Hillis et al. (1993) presented analyses of anuran relationships based on DNA sequence data from the mitochondrial and nuclear ribosomal genes, respectively.
Hedges et al. (1990) also analyzed 35 variable sites from 28S rRNA of four species of frogs, from Discoglossidae, Hylidae, Pelobatidae, and Pipidae. Bootstrap analysis of maximum parsimony trees yielded a monophyletic Anura, but with no resolution among the four taxa. The neighbor-joining analysis indicated a sister-group relationship between the discoglossid and pipid, and between the bufonid and hylid, but the bootstrap value for both of these nodes was less than 50%. Neither analysis yielded a monophyletic Amphibia.
Hay et al. (1995) is the most comprehensive molecular systematics treatment of the relationships among the families of amphibians. Their neighbor-joining tree yielded a monophlyletic Anura, Caudata, and Gymnophiona.
"Archaeobatrachia"
Archaeobatrachians have generally included discoglossoids, pipoids and pelobatoids (Duellman, 1975; Reig, 1958). The synapomorphies discussed below for Leiopelmatanura, Bombinanura, Discoglossanura, and Pipanura collectively indicate that "Archaeobatrachia" is paraphyletic. The informal term archaeobatrachian is a convenient term for anurans that are not part of Neobatrachia.
Leiopelmatanura (Node B)
This new node-based name was applied by Ford and Cannatella (1993) to the node that is the most recent ancestor of living Leiopelma + Bombinanura (Ford and Cannatella, 1993). Synapomorphies of this clade (Cannatella, 1985) include elongate arms on the sternum; loss of the ascending process of the palatoquadrate; sphenethmoid ossifying in the anterior position; the root of the facial nerve exits the braincase through the facial foramen, anterior to the auditory capsule, rather than via the anterior acoustic foramen into the auditory capsule (Slabbert and Maree, 1945; Stephenson, 1951); and a palatoquadrate articulation with the braincase via a pseudobasal process, rather than a basal process (Pusey, 1943).
Bombinanura (Node C)
Bombinanura, a new node-based name, was defined by Ford and Cannatella (1993) to be the most recent common ancestor of living Bombinatoridae and Discoglossanura, and all its descendants. Synapomorphies of Bombinanura include fusion of the halves of the sphenethmoid, eight presacral vertebrae, absence of the m. epipubicus, and absence of the caudalipuboischiotibialis muscle (Cannatella, 1985). Subclades of Bombinanura include Bombinatoridae and Discoglossanura.
"Discoglossidae"
Most workers have placed Alytes, Discoglossus, Bombina, and Barbourula in the family Discoglossidae although the dissimilarity of Alytes and Discoglossus, on one hand, and Bombina on the other has often been noted (e.g., Lanza et al., 1976). There are apparently no published synapomorphies for "Discoglossidae", and synapomorphies of Discoglossanura (below) reject the monophyly of "Discoglossidae" as traditionally used. Griffiths (1963) stated that the diagnostic feature of "Discoglossidae" (including Bombinatoridae and Discoglossidae) is a triradiate sternum. However, this type of sternum is also present in Leiopelma, and is interpreted as a synapomorphy of Leiopelmatanura.
Discoglossanura (Node D)
The new node-based name Discoglossanura is defined as the most recent common ancestor of living Discoglossidae + Mesobatrachia + Neobatrachia, and all its descendants. Synapomorphies of Discoglossanura include a bicondylar sacrococcygeal articulation and the presence of an episternum. Discoglossanura has two subgroups, Discoglossidae and Pipanura.
Saint-Aubain (1981) listed synapomorphies from the ontogeny of the carpus that suggested that Ascaphus, Leiopelma, bombinatorids, discoglossids, and pipids form a clade. However, her sampling of taxa is not sufficient to provide strong support for this clade, especially in light of the synapomorphies supporting other arrangements.
Pipanura (Node E)
The node-based name Pipanura was proposed by Ford and Cannatella (1993) for the most recent common ancestor of living Mesobatrachia + Neobatrachia, and all its descendants. The subordinal name Ranoidei was coined for this clade by Sokol (1977), but that name was re-assigned to a less inclusive taxon by Dubois (1983, 1984). Sokol's (1977) use of this name was unfortunate because the informal name, ranoid, is homonymous with the widely used name Ranoidea.
Synapomorphies include a sinistral spiracle in the larvae (a characteristic feature of Orton's Type 4 tadpole), absence of free ribs in adults, torsion in the carpal elements, the presence of vocal sacs, and fusion of the trigeminal and facial ganglia (Cannatella, 1985; Sokol, 1975).
Mesobatrachia (Node F)
Ford and Cannatella (1993) applied the name Mesobatrachia to the node that is the most recent common ancestor of the living Pelobatoidea and Pipoidea. Synapomorphies of Mesobatrachia include closure of the frontoparietal fontanelle by juxtaposition of the frontoparietal bones, partial closure of the hyoglossal sinus by the ceratohyals (=hyalia), absence of the taenia tecti medialis, and absence of the taenia tecti transversum (Cannatella, 1985; Sokol, 1981). Mesobatrachia as proposed by Laurent (1979) was a paraphyletic group. Cannatella (1985) first applied the name to a clade. Most other taxonomies have placed the Pelobatoidea as the sister-group to Neobatrachia, rather than to Pipoidea. A derived character that refutes the monophyly of Mesobatrachia is the fusion of the third distal carpal to other carpals, which Pelodytes shares with Neobatrachia (Cannatella, 1985).
Pelobatoidea (Node G)
The name was applied to the node that is the common ancestor of living Megophryidae, Pelobatidae, and Pelodytes. Synapomorphies of Pelobatoidea include the presence of a palatine process of the maxilla and ossification of the sternum into a bony style (Cannatella, 1985). Duellman and Trueb (1986) listed the presence of a dorsal gap in the cricoid ring as a synapomorphy for this clade. However, there is no gap in Scaphiopus, Spea, Pelobates (except for the smallest species, P. fuscus), or several megophryids; at best, the presence of a dorsal gap would be an ambiguous synapomorphy. Relationships among the living pelobatoids are an unresolved trichotomy of Megophryidae, Pelobatidae, and Pelodytes.
Pipoidea (Node H)
Pipoidea was defined by Cannatella and Ford (1993) to be the most recent common ancestor of living Pipidae + Rhinophrynidae, and all its descendants. Pipoidea is diagnosed by several distinctive synapomorphies, including the absence of mentomeckelian bones (see comments below on †Palaeobatrachidae), absence of lateral alae of the parasphenoid, fusion of the frontoparietals into an azygous element, greatly enlarged otic capsules, and a tadpole with paired spiracles, and lacking beaks and denticles (Orton Type 1 tadpole). Clades of Pipoidea are Pipidae, †Palaeobatrachidae, some unplaced fossils "pipids" and Rhinophrynidae.
Pipimorpha (Stem I)
The new stem-based name Pipimorpha was defined by Ford and Cannatella (1993) to be those taxa that are more closely related to living Pipidae than to living Rhinophrynus. Pending a more detailed assessment of the relationships of †Palaeobatrachidae, Pipidae, and the fossil "pipids," we consider †Palaeobatrachidae, †Thoraciliacus, †Cordicephalus, †Saltenia, †Shomronella, and †Eoxenopoides to be part of Pipimorpha.
References
Click here for more detailed references.
Cannatella, D. C. 1985. A phylogeny of primitive frogs (archaeobatrachians). Ph.D. Dissertation, The University of Kansas, Lawrence.
Cannatella, D. C. 1989. On the monophyly of discoglossoid frogs. Pp. 230-231 In H. Splechtna and H. Hilgers (Eds.), Trends in Vertebrate Morphology. Gustav Fischer Verlag, Stuttgart.
Cannatella, D. C., and D. M. Hillis. 1993. Amphibian phylogeny: phylogenetic analysis of morphology and molecules. Herpetol. Monogr. 7:1-7.
de Queiroz, K., and J. Gauthier. 1992. Phylogenetic taxonomy. Ann. Rev. Ecol. Syst. 23:449-80.
Dubois, A. 1983. Classification et nomenclature supragénérique des amphibiens anoures. Bull. Soc. Linn. Lyon 52:270-276.
Dubois, A. 1984. La nomenclature supragénérique des amphibiens anoures. Mémoires Museum Nat. d'Histoire Naturelle, Ser.A 131:1-64.
Duellman, W. E. 1975. On the classification of frogs. Occ. Pap. Mus. Nat. Hist. Univ. Kansas (42):1-15.
Duellman, W. E., and L. Trueb. 1986. Biology of Amphibians. McGraw-Hill Book Co., New York.
Estes, R., and O. A. Reig. 1973. The early fossil record of frogs: a review of the evidence. Pp. 11-63 In J. L. Vial (Ed.), Evolutionary Biology of the Anurans: Contemporary Research on Major Problems. University of Missouri Press, Columbia.
Ford, L. S. 1989. The phylogenetic position of poison-dart frogs (Dendrobatidae): reassessment of the neobatrachian phylogeny with commentary on complex character systems. Ph.D. Dissertation, The University of Kansas, Lawrence, Kansas.
Ford, L. S., and D. C. Cannatella. 1993. The major clades of frogs. Herp. Monogr. 7:94-117.
Frost, D. R. (Ed.) 1985. Amphibian Species of the World. Allen Press and the Association of Systematics Collections, Lawrence, Kansas.
Gans, C., and T. S. Parsons. 1966. On the origin of the jumping mechanism in frogs. Evolution 20(1):92-99.
Griffiths, I. 1963. The phylogeny of the Salientia. Biological Reviews 38:241-292.
Halliday, T. R., and K. Adler (Eds.). 1987. The Encyclopedia of Reptiles and Amphibians. Equinox, Oxford.
Hay, J. M., I. Ruvinsky, S. B. Hedges, and L. R. Maxson. 1995. Phylogenetic relationships of amphibian families inferred from DNA sequences of mitochondrial 12S and 16S ribosomal RNA genes. Mol. Biol. Evol. 12(5):928-937.
Hedges, S. B., K. D. Moberg, and L. R. Maxson. 1990. Tetrapod phylogeny inferred from 18s and 28s ribosomal RNA sequences and a review of the evidence for amniote relationships. Mol. Biol. Evol. 7:607-633.
Hedges, S. B. and L. R. Maxson. 1993. A molecular perspective on lissamphibian phylogeny. Herpetol. Monogr. 7:27-42.
Hillis, D. M., L. K. Ammerman, M. T. Dixon, and R. O. de Sá. 1993. Ribosomal DNA and the phylogeny of frogs. Herpetol. Monogr. 7:118-131.
Inger, R. F. 1967. The development of a phylogeny of frogs. Evolution 21:369-384.
Kluge, A. G., and J. S. Farris. 1969. Quantitative phyletics and the evolution of anurans. Syst. Zool. 18:1-32.
Lanza, B., J. M. Cei, and E. G. Crespo. 1976. Further immunological evidence for the validity of the family Bombinidae (Amphibia, Salientia). Monit. Zool. Ital. (NS) 10:311-314.
Laurent, R. F. 1979. Esquisse d'une phylogenèse des anoures. Bull. Soc. Zool. France 104:397-422.
Laurent, R. F. 1986. Sous classe des lissamphibiens. Systématique. Pp. 594-797 In P.-P. Grassé and M. Delsol (Eds.), Traité de Zoologie, Tome 14, 1B. Masson, Paris.
Lynch, J. D. 1971. Evolutionary relationships, osteology, and zoogeography of leptodactyloid frogs. Misc. Publ. Mus. Nat. Hist. Univ. Kansas (53):531-238.
Lynch, J. D. 1973. The transition from archaic to advanced frogs. Pp. In J. L. Vial (Ed.), Evolutionary Biology of the Anurans: Contemporary Research on Major Problems. University of Missouri Press, Columbia.
Milner, A. R. 1988. The relationships and origin of living amphibians. Pp. 59-102 In M. J. Benton (Ed.), The Phylogeny and Classification of the Tetrapods. 1. Amphibians, Reptiles, Birds. Oxford University Press, Oxford.
Milner, A. R. 1993. The Paleozoic relatives of lissamphibians. Herpetol. Monogr. 7:8-27.
Noble, G. K. 1922. The phylogeny of the Salientia. I. The osteology and the thigh musculature; their bearing on classfication and phylogeny. Bull. Amer. Mus. Nat. Hist. 46:1-87.
Noble, G. K. 1931. The Biology of the Amphibia. McGraw-Hill, New York.
Orton, G. 1957. The bearing of larval evolution on some problems in frog classification. Syst. Zool. 6:79-86.
Peters, J. A. 1964. Dictionary of Herpetology. Hafner Publishing Co., New York.
Pusey, H. K. 1943. On the head of the liopelmid frog, Ascaphus truei. I. The chondrocranium, jaws, arches, and muscles of a partly grown larva. Quart. J. Micr. Sci. 84:106-185.
Reig, O. A. 1958. Proposiciones para una nueva macrosistematica de los anuros. Nota preliminar. Physis 21:109-118.
Rocek, Z. 1981. Cranial anatomy of frogs of the family Pelobatidae Stannius, 1856, with outlines of their phylogeny and systematics. Acta Univ. Carolinae Biol. 1980:1-164.
Saint-Aubain, M. L. de 1981. Amphibian limb ontogeny and its bearing on the phylogeny of the group. Zeit. Zool. Systematik u. Evolutionsforschung 19:175-194.
Savage, J. M. 1973. The geographic distribution of frogs: patterns and predictions. Pp. 351-445 In J. L. Vial (Ed.), Evolutionary Biology of the Anurans: Contemporary Research on Major Problems. University of Missouri Press, Columbia.
Slabbert, G. K., and W. A. Maree. 1945. The cranial morphology of the Discoglossidae and its bearing upon the phylogeny of the primitive Anura. Ann. Univ. Stellen. 23a:91-97.
Sokol, O. M. 1975. The phylogeny of anuran larvae: a new look. Copeia 1975:1-24.
Sokol, O. M. 1977a. A subordinal classification of frogs (Amphibia: Anura). J. Zool., London 182:505-508.
Starrett, P. H. 1968. The phylogenetic significance of the jaw musculature in anuran amphibians. PhD. Dissertation. University of Michigan, Ann Arbor, Michigan.
Starrett, P. H. 1973. Evolutionary patterns in larval morphology. Pp. 251-271. In J. L. Vial (Ed.), Evolutionary Biology of the Anurans: Contemporary Research on Major Problems. University of Missouri Press, Columbia.
Stephenson, E. M. T. 1951. The anatomy of the head of the New Zealand frog, Leiopelma. Trans. Zool. Soc. London 27:255-305.
Trueb, L. 1973. Bones, frogs, and evolution. Pp. In (Eds.), In: Evolutionary Biology of the Anurans: Contemporary Research on Major Problems (J.L. Vlal, ed.), pp. 65-132. Univ. of Missouri Press, Columbia.
Trueb, L., and R. Cloutier. 1991. A phylogenetic investigation of the inter- and intrarelationships of the Lissamphibia (Amphibia: Temnospondyli). Pp. 233-313. In H.-P. Schultze and L. Trueb (Eds.), Origins of the Higher Groups of Tetrapods. Cornell University Press, Ithaca.
Trueb, L. 1993. Patterns of cranial diversity among the Lissamphibia. Pp. 255-343 In J. Hanken and B. Hall (Eds.) The Vertebrate Skull. University of Chicago Press, Chicago.
Information on the Internet
- AmphibiaTree. The mission of this project is the realization of a comprehensive phylogeny of all taxa of extant and extinct amphibians.
- AmphibiaWeb. A site inspired by global amphibian declines, is an online system that allows free access to information on amphibian biology and conservation.
- Amphibian Species of the World. American Museum of Natural History.
- FrogWeb: Amphibian Declines & Deformities. National Biological Information Infrastructure.
- Froglog. Newsletter of the Declining Amphibian Populations Task Force of the World Conservation Union's Species Survival Commission.
- FROGS.ORG. Website of the Amphibian Conservation Alliance.
- Frogs. A Chorus of Colors. American Museum of Natural History.
- Exploratorium: Frogs. An exhibition at the Exploratorium, the museum of science, art and human perception.
- The Frog Files. Information on the frogs of Western Australia.
- MidWest Frogs. Frog call video clips.
- Frogs Home. University of Wisconsin Sea Grant Institute.
- A Thousand Friends of Frogs. Connecting children, parents, educators, and scientists to study and celebrate frogs and their habitats. Center for Global Environmental Education, Hamline University.
- Frogs and Toads in Color and Sound. Lang Elliott, NatureSound Studio.
- Manitoba Frog and Toad Calls.
- Virtual frog dissections:
- Net Frog. Frog dissection and anatomy
- Froguts. The first true virtual online frog dissection.
- LBL ITG Whole Fro Project. Interactive frog dissection kit.
Title Illustrations
| Scientific Name | Ascaphus truei |
|---|---|
| Location | Oregon |
| Specimen Condition | Live Specimen |
| Sex | Male |
| Copyright | © 1995 David Cannatella |
About This Page
Production of the Salientia clade of the Tree of Life was funded in part by Project QUEST, the Texas Memorial Museum, and the College of Natural Sciences of the University of Texas, Austin, Texas.
David Cannatella
Section of Integrative Biology and Texas Memorial Museum, University of Texas, Austin, TX 78712
Department of Herpetology, American Museum of Natural History, Central Park West at 79th St. New York, NY 10024
Department of Zoology, University of Texas, Austin, TX 78712
Correspondence regarding this page should be directed to David Cannatella at
Page copyright © 1997 David Cannatella
Citing this page:
Cannatella, David, Ford, Linda, and Bockstanz, Lori. 1997. Salientia. Frogs and toads. Version 01 January 1997 (under construction). http://tolweb.org/Salientia/14938/1997.01.01 in The Tree of Life Web Project, http://tolweb.org/