Clusiidae
Owen Lonsdale and Steve Marshall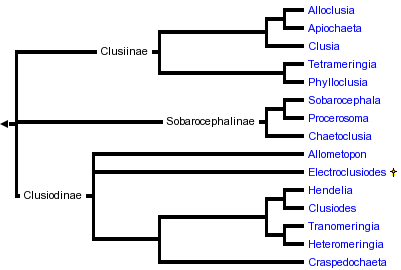

This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
The Clusiidae are a family of small, thin, yellow to black flies with the wing usually partially infuscated. The antenna most readily diagnoses the family: the outer and (sometimes) inner distal margins of the pedicel have a triangular extension, and the arista is dorsoapical (separating it from most other acalyptrate families) and pubescent to densely plumose or laterally flattened.
Adults are uncommonly collected in the field, but they can be relatively abundant in some microhabitats. Small dung baits and Malaise traps have been the most successful methods of collection, but a number of species have been swept from grass or collected off of foliage, logs and dead patches of wood on standing trees. North American adults appear to prefer mixed and deciduous forests sometimes associated with grass-dominated areas. Tropical species have often been collected along waterways in mossy, humid habitats. Clusiids have been known to feed on nectar, rotting vegetative matter, sap (So?s 1987), and the dung of birds and mammals.
The Clusiidae are one of a handful of acalyptrate families known to engage in lekking behaviour. Males establish dominance in a lekking site by defending territories (devoid of resource) from other males on logs or branches in order to attract females and mate. After mating, clusiid females lay eggs elsewhere, usually under bark or in wood in a state of more advanced decay than the surface used for lekking (Roh?cek 1995). The wood likely provides a moist environment with an adequate supply of saprobes for feeding larvae. Selection of clusiid oviposition sites does not appear to be associated with any particular species of tree, but is limited by "humidity, amount of shade, stage of wood decay, [and the] presence of mycelia of certain fungi" (Roh?cek 1995). Larvae of Sobarocephala occur in the same environment as the adults, and have been found only within decaying wood and termite colonies (Soos 1987). Malloch (1918) also found the larvae of S. flaviseta to be "evidently associated with the burrows of coleopterous insects" and to be relatively sluggish and slow moving.
Males of some clusiids, particularly Hendelia Czerny, have enlarged heads or other conspicuous modifications used in mutual assessment on lek sites (McAlpine 1976, Marshall 2000). Sobarocephala latipennis Melander & Argo and several Australian Hendelia have strongly widened heads, and Hendelia kinetrolicros (Caloren & Marshall), Hendelia mirabilis (Frey), and Procerosoma alini (Shatalkin) have spectacular long genal processes that are probably used in male-male agonistic interactions, although these species have never been observed while engaged in such behavior.
Characteristics
Clusiids are slender acalyptrate flies 2.5-6.0 mm in length, most readily identified by an angulate extension on the outer margin of the pedicel, a dorsoapical arista (dorsobasal in similar families), a complete subcosta, one subcostal break, one pair of vibrissae, and five or fewer fronto-orbital bristles. Most species are yellow with a brown to black pattern, but several species are entirely pale (some Sobarocephala Czerny), and a number of taxa are predominantly brown to black (many Heteromeringia Czerny and Craspedochaeta Czerny).
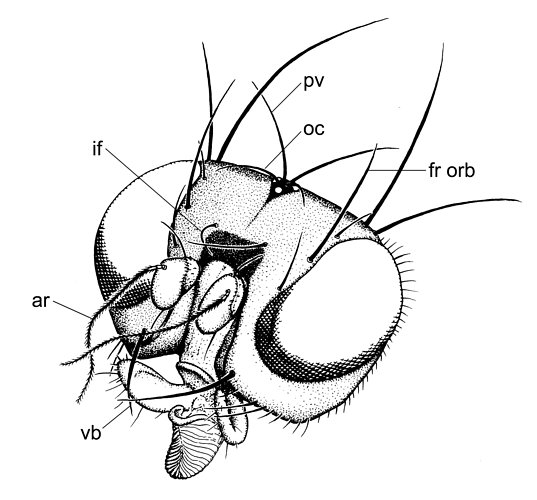 image info
image info
Head of Clusiodes johnsoni Malloch; (ar) arista, (fr orb) fronto-orbital bristle, (if) interfrontal bristle, (oc) ocellar bristle, (pv) postvertical bristle, (vb) vibrissae. Drawing ? Owen Lonsdale.
There are three to five (sometimes two) fronto-orbital bristles; the anterior bristle is sometimes inclinate, and if there are four or five fronto-orbitals, the third from the back is usually inclinate and proclinate (Craspedochaeta). Convergent interfrontal bristles are sometimes present. The ocellar and postvertical bristles are divergent and usually small to absent. On the thorax, there are one to three dorsocentral bristles, sometimes one presutural acrostichal bristle, one postpronotal, two notopleurals, two or three intra-alars (the presutural intra-alar is sometimes absent), one or two intra post-alar bristles, and one strong anepisternal and katepisternal bristle. The mid and hind tibiae sometimes have one or two dorsal preapical bristles. The subcosta is complete and a subcostal break is present, although this break is indistinct in most Clusiodinae (Lonsdale & Marshall in press, b).
Eggs are translucent, usually three to four times longer than wide, and approximately as long as sternite 6 of the female (Lonsdale & Marshall in press, b). Both ends are tapered (most pronounced anteriorly) with the micropyle small and terminal. The surface of the egg is minutely tuberculate with (usually) no more than a dozen longitudinal wrinkles. In Sobarocephala, these wrinkles are bordered by larger quadrate tubercles, and some of the wrinkles are branched.
Larvae are known from European Clusiodes Coquillett. The cephalic papillae are minute, the mandibles are small and well sclerotized, and the cephalopharyngeal skeleton is vestigial and unpigmented (So?s 1987). The anterior spiracles have six openings, each on a small raised tubercle (So?s 1987). The posterior spiracles are elevated on chitinized plates, produced as dorsally curving hooks with three elongate-oval openings on the medial or ventral half (Malloch 1918). The anal plate is large, heavily sclerotized, wider than long and tapered laterally (Malloch 1918).
Puparia (known from temperate Clusia Haliday, Sobarocephala and Clusiodes) are brownish-orange in colour and covered with numerous minute transverse wrinkles (Lonsdale & Marshall in press, b). The posterior face is smooth with various sculpturing, and either sharply curved and bordered by a high ridge (Clusia and Sobarocephala), or broadly rounded with the ridge broken and shallow (Clusiodes). The anal hooks curve dorsally (Clusiodes) or dorsomedially, and are ovate (Clusia) or semi-circular (Sobarocephala) in cross-section (Lonsdale & Marshall in press, b).
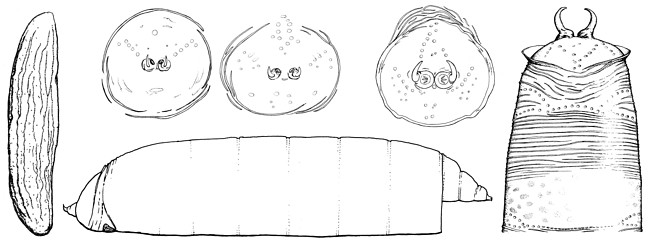 image info
image info
Figure: Immature stages clockwise from left: egg, Clusiodes pictipes (Zetterstedt); puparium (posterior face), Clusiodes johnsoni Malloch; puparium (posterior face), Clusiodes melanostomus (Loew); puparium (posterior face), Clusia occidentalis Malloch; puparium (dorsal - posterior third), Clusia occidentalis; puparium (lateral) Clusiodes johnsoni. ? Owen Lonsdale.
Externally, clusiid male genitalia are composed of a dome-shaped epandrium, well developed surstyli, two cerci that are confluent basally, and an 'annulus' comprised of sternites 6-8 (Lonsdale & Marshall in press, a). Internally, the genitalia are made up of a subepandrial sclerite, a hypandrium with three lateral setae and a pair of 'arms' that attach to the subepandrial sclerite, a rod-like phallapodeme, a ring-like basiphallus, a fin-like epiphallus, a short to long distiphallus, one pair of lateral lobes at the base of the distiphallus, a single ejaculatory apodeme, and one pair of pregonites and postgonites. This ground-plan is highly modified in the Clusiodinae excluding Allometopon Kertesz, as the postgonite, epiphallus, and lateral lobes of the distiphallus are absent, the pregonite is enlarged and fused to the hypandrium, the phallapodeme is wide with a dorsal "fin", and the hypandrial arms are partly fused to the annulus (Lonsdale & Marshall, in press, a). The phallus itself is also highly modified, being either large and sac-like, extremely long and coiled, or atrophied to absent.
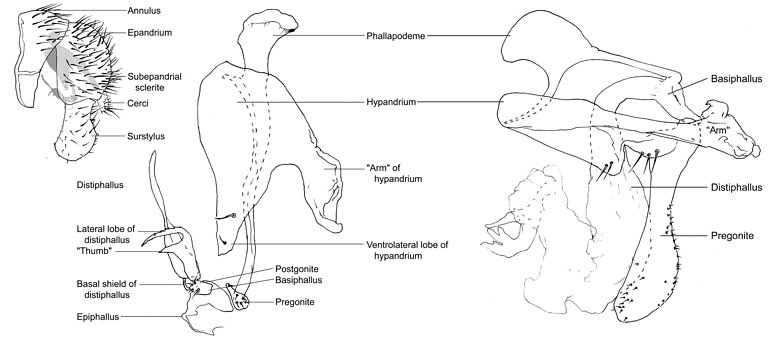 image info
image info
Figure. Top left: Sobarocephala flava male terminalia, left lateral (hypandrial complex shaded); centre: Sobarocephala flava hypandrial complex, left lateral; Clusiodes apicalis hypandrial complex, left lateral. ? Owen Lonsdale.
The female genitalia are much more simple than those of the male, being comprised of a ventral receptacle and one pair of spermathecae. These structures are relatively poorly sclerotized and difficult to examine, and staining is often necessary. In the Clusiinae and Sobarocephalinae, the spermathecae are simple and spherical, but in the Clusiodinae, they are longitudinally segmented and sometimes telescoped and heavily pigmented. The ventral receptacle is variably elaborated in some Clusiodinae.
Discussion of Phylogenetic Relationships
Frey (1960) divided the Clusiidae into two subfamilies, Clusiodinae (characterized by reclinate fronto-orbital bristles) and Clusiinae (with at least one pair of inclinate fronto-orbital bristles). Subsequent authors, including Steyskal (1965), So?s (1987), Sasakawa (1977) and Pitkin & Evenhuis (1989) restricted the Clusiinae to those genera with only the anterior fronto-orbital bristle inclinate, moving Craspedochaeta to the Clusiodinae. Neither of these subfamily concepts based on head chaetotaxy correspond with clades defined by numerous synapomorphies of the male and female genitalia and previously unrecognized external morphological characters. Three subfamilies are now recognized within the Clusiidae: Clusiodinae, Clusiinae and Sobarocephalinae (Lonsdale & Marshall in press, a). The relationships between these subfamilies are currently unresolved.
The Clusiodinae contains seven genera (Allometopon, Clusiodes, Craspedochaeta, Hendelia, Heteromeringia, Tranomeringia Sasakawa, and the fossil genus Electroclusiodes Hennig (Lonsdale & Marshall, in manuscript)) defined by longitudinally segmented spermathecae, loss of the ventrolateral lobes of the hypandrium, entirely reclinate fronto-orbital bristles and loss of the presutural intra-alar bristle (the latter two characters are of uncertain polarity) (Lonsdale & Marshall in press, a). An inclinate anterior fronto-orbital is recovered in Heteromeringia and the Indoaustralian Tranomeringia, which had previously classified them outside of the subfamily.
Excluding Heteromeringia and Tranomeringia, the Clusiidae with inclinate anterior fronto-orbital bristles can be divided into two clades, the subfamilies Clusiinae and Sobarocephalinae (Lonsdale & Marshall in press, a). The Sobarocephalinae includes the large, primarily neotropical genus Sobarocephala and two small, closely related neotropical genera (Chaetoclusia Coquillett and Procerosoma Lonsdale & Marshall). The Sobarocephalinae is a poorly-supported clade defined by male genitalic characters: loss of the sixth spiracle (otherwise left lateral on the annulus), a ventral lobe of the hypandrium that is usually at least as long as the hypandrial arm (secondarily reduced in several species of Chaetoclusia and Sobarocephala), and a relatively large surstylus.
The Clusiinae includes the southern South American genera Alloclusia and Apiochaeta, the small Eurasian and Australian genera Phylloclusia Hendel, Tetrameringia McAlpine and Paraclusia Czerny and the Holarctic and Oriental genus Clusia (Lonsdale & Marshall in press, a). The subfamily is more well supported than the Sobarocephalinae, defined by a small ratio of the length of the ultimate section of vein M to the penultimate, outstanding bristles on the posterodorsal surface of the fore femur, an acute process on the inner-basal face of the surstylus, a bent or jointed distiphallus, and a posteromedial truncated notch on the vertex.
References
Frey, R. 1960. Studien ?ber indoaustralische Clusiiden (Dipt.) nebst Katalog der Clusiiden. Commentationes Biologicae. 22(2): 1-31.
Lonsdale, O. & Marshall, S.A. In press, a. Redefinition of the Clusiinae and Clusiodinae, description of the new subfamily Sobarocephalinae, revision of the genus Chaetoclusia and a description of Procerosoma gen. nov. (Diptera: Clusiidae). European Journal of Entomology.
Lonsdale, O. & Marshall, S.A. In press, b. Family Clusiidae In. Diptera of Central America, Brown, B (ed.).
Malloch J.R. 1918: A revision of the dipterous family Clusiodidae (Heteroneuridae). Proceedings of the Entomological Society of Washington 20(1): 2-8.
Marshall, S.A. 2000. Agonistic behaviour and generic synonymy in Australian Clusiidae (Diptera). Studia Dipterologica. 7: 3-9.
McAlpine, D.K. 1976. Spiral vibrissae in some clusiid flies (Diptera: Schizophora). Australian Entomological Magazine. 3(4): 75- 78.
Pitkin, B.R. & N.L. Evenhuis. 1989. Family Clusiidae. In N.L. Evenhuis (editor). Catalog of the Diptera of the Australasian and Oceanian regions. Pp. 534-536. Bishop Museum Press & E.J. Brill, Honolulu.
Sasakawa, M. 1977. Family Clusiidae. In M.D. Delfinado & D.E. Hardy (editors). A catalog of the Diptera of the Oriental region. Pp. 234-239. University Press of Hawaii, Honolulu.
Steyskal, G.C. 1965. Family Clusiidae. In Stone et al. (eds.). A catalogue of the Diptera of America north of Mexico. U.S. Dept. of Agriculture, Agricultural Research Service, Washington, D.C.
Roh?cek, J. 1995. Clusiidae (Diptera) of the Czech and Slovak Republics: Faunistics and notes on biology and behaviour. Casopis Slezsk?ho Muzea Opava. (A) 44: 123-140.
S?os A. 1987: Clusiidae McAlpine J.F. (ed.): Manual of Nearctic Diptera, Vol. 2. Monograph 28, Research branch, Agriculture Canada, Ottawa Pp. 853-857.
Title Illustrations
| Scientific Name | Alloclusia limbipennis (Rondani) |
|---|---|
| Location | Chile |
| Specimen Condition | Live Specimen |
| Life Cycle Stage | Adult |
| Copyright | © 2004 Steve Marshall |
| Scientific Name | Hendelia gladiator (McAlpine) |
|---|---|
| Location | Australia |
| Specimen Condition | Live Specimen |
| Sex | Male |
| Copyright | © 2004 Steve Marshall |
About This Page
Owen Lonsdale
Insect Systematics Lab
Department of Environmental Biology
University of Guelph
Guelph, ON
N1G 2W1
Canada
Steve Marshall
Insect Systematics Lab
Department of Environmental Biology
University of Guelph
Guelph, ON
N1G 2W1
Canada
Correspondence regarding this page should be directed to Owen Lonsdale at and Steve Marshall at
Page copyright © 2004 Owen Lonsdale and Steve Marshall
- First online 10 December 2004
Citing this page:
Lonsdale, Owen and Marshall, Steve. 2004. Clusiidae. Version 10 December 2004 (under construction). http://tolweb.org/Clusiidae/10628/2004.12.10 in The Tree of Life Web Project, http://tolweb.org/