Vampyromorpha
Vampyroteuthis infernalis
The Vampire Squid
Richard E. YoungIntroduction
The vampire squid is rather small, reaching a maximum of 13 cm ML (Nesis, 1982/7), and is very gelatinous; its consistency is that of a jellyfish. It occupies meso- to bathypelagic depths throughout the tropical and temperate regions of the world's oceans. The second pair of arms is modified into retractile filaments that can extend to lengths well in excess of the total length of the animal, and they can be retracted into pockets within the web. The filaments, presumably, have a sensory function. The vampire has black chromatophores with reddish-brown ones interspersed. These chromatophores, however, have lost the muscles that enable rapid color change in other coleoids and are probably incapable of changing shape. A few normal chromatophores associated with photophores are still present.
The vampire is a phylogenetic relict and possesses features of both octopods and decapods. In addition, it has many features that are probably adaptations to the deep-sea environment. Among these are the loss of the ink sac and most active chromatophores, development of photophores and the gelatinous consistency of the tissues.
Brief diagnosis:
An octopodiform ...
- with arms II modified as slender filaments.
- with cirri but without suckers on the proximal halves of arms.
Characteristics
- Arms
- "Arms II" are retractile filaments.
- Lateral cirri present over arm length; suckers present only on distal half of arms where they alternate with cirri.
- Suckers without cuticular lining; sucker stalks constricted to narrow plate at base of sucker.
 Click on an image to view larger version & data in a new window
Click on an image to view larger version & data in a new window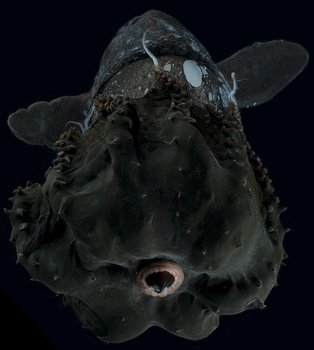
Figure. Ventro-oral view of V. infernalis with arms folded aborally, showing cirri without associated suckers in proximal half of arms. Note the sparse red pigment and the colorless, oral tips of the arms. Photographed in a shipboard aquarium prior to fixation by
- Head
- Nuchal cartilage present.
- Central nervous system with incipient inferior frontal lobe system; superior buccal lobes adjacent (fused at edges) to posterior buccal lobes; suprabrachial commissure lies within brain.
- Funnel
- Funnel valve absent.
- Funnel valve absent.
- Mantle
- Dorsal mantle cavity absent.
- Dorsal mantle cavity absent.
- Fins
- Two pairs of fins present during ontogeny.
- Two pairs of fins present during ontogeny.
- Spermatangia
- Receptacle (deep sac) for spermatangia located anterior to each eye in females.
- Receptacle (deep sac) for spermatangia located anterior to each eye in females.
- Shell
- Shell a gladius with broad median field and broad conus.
- Shell a gladius with broad median field and broad conus.
- Photophores
- Large circular, lidded organs present posterior to each adult fin ("fin-base" organs).
- Numerous small organs distributed over ventral surfaces of mantle, funnel, head and aboral surface of arms and web ("skin-nodule" organs). Two patches on dorsal surface of head look like aggregrated small photophores but are photoreceptors (Herring, et al., 1994).
- Arm-tip organ(s) produce luminescent clouds consisting of microscopic glowing particles (Robison, et al., 2003).
- Arm-tip organs that flash or glow (Hunt, 1996).
Insitu photographs of the vampire squid showing the "skin-nodule" photophores can be seen here.
- Viscera
- Oviducal glands at terminal end of oviducts.
- Visceropericardial coelom extends posteriorly as a slender duct, possibly a remnant siphuncle.
- Photosensitive vesicles located immediately dorsal to funnel and, possibly, on dorsal integument in nuchal region.
Comments
A drawing and photographs of the mantle cavity can be seen here.
Nomenclature
A list of all nominal genera and species in the Vampyroteuthidae can be found here. The list includes the current status and type species of all genera, and the current status, type repository and type locality of all species and all pertinent references.
Life History
- Fins
- Development of the fins in the vampire is unique among cephalopods. One pair is present at hatching and is eventually resorbed and replaced by a more anterior pair as development proceeds. At one stage in development, therefore, the vampire has two pairs of fins. The first pair to develop is the homologue of the fins of other cephalopods (Young and Vecchione, 1996). The unusual fin ontogeny is partially responsible for the early description of 3 families and many species where only one species actually exists. Except for the fins, the young vampire squid (ca 10 mm ML) have an appearance very similar to that of the adult.
- Eggs and hatchlings
- Vampires lack nidamental glands and have rather small oviducal glands. As a result there is little likelihood that they produce large egg masses. Off California small vampire squid occupy greater depths than do the larger individuals (Roper and Young, 1975) suggesting that spawning occurs in very deep water. A hatchling is known from deep water off Hawaii.
Behavior
Vampyroteuthis can swim surprisingly fast for a gelatinous animal. Hunt (1996) estimates from videotapes that it can reach two body lengths/sec, and it can accellerate to this speed in 5 sec. An escape reaction involves the quick movement of the fins toward the funnel followed by a jet from the mantle. This sequence is repeated as the vampire takes a series of quick turns in an erratic escape route (Hunt, 1996). The arms are sometimes spread forward to form, along with the web, an umbrella-like or bell-shaped posture while the vampire slowly swims forward (Hunt, 1996). The vampire appears to orient most commonly in a horizontal attitude with generally one filament extended (Hunt, 1996). The filaments appear to be tactile sense organs (Hunt, 1996). It has a posture ("pineapple posture") in which the arms and web are spread aborally over the head and mantle (Robison, 1995). In this posture the squid would be somewhat more difficult to injure and would be covered by a densely pigmented cloak. The oral surface of the arms and webs are the most heavily pigmented (black) regions on the animal. The posture, therefore, is probably a defensive one.


Arm tip from a preserved vampire squid showing the light emitting surface (unpigmented), as well as suckers and cirri (photograph copyright © 1999, R. E. Young).
Hunt (1966) first observed bioluminescence displays in the living animal. Fin-base photophores have been observed to glow brightly for less than a second (a flash) or longer than two minutes. In addition the light intensity can vary giving a pulsating appearance, and as light is extinguished, the glowing disc can be seen to decrease in diameter as well as intensity.
Arm-tip organs are unpigmented on their oral surface where light is emitted but otherwise do not look like luminescent structures (see photograph on the right). The photophores all glow simultaneously, or they all can flash at a rate of one to three per second or pulsate. With the arm-tip organs apparently glowing continuously, the vampire moves the arms ("arm writhing") around rapidly exposing and hiding the photophores which is "...very disorienting [to an observer] when trying to visually fix the animal's position" (Hunt, 1996 p. 104). Often a flash of the arm tips is followed by a rapid escape response. Another unusual and visually confusing effect is seen when viewing the vampire posteriorly from the mantle apex. The apparently disturbed vampire can curl the arms and web posteriorly over the head ("pineapple posture") then illuminate the arm-tip organs and the fin-base organs. "...the arm tips appear to come toward you, whereas the fin[base]lights appear to be moving away (due to their apparent shrinkage)" (Hunt, 1996, p. 104).
The third source of bioluminescence is luminescent clouds. These appear as a mucous matrix with a few hundred to over 1000 discrete, glowing particles embedded in it. The particles can glow for up to 9.5 min. The source of the particles has been shown to be the arm tip organs (Robison, et al., 2003). The latter authors suggest that flashing of the arm-tip organs is controlled by covering or exposing the photogenic region by the pigmented sides of the arm tips, that the microscopic glowing particles are not luminescent bacteria, and that the function of the bioluminescence is to startle or distract a predator.
Some spectacular videos of Vampyroteuthis bioluminescence can be seen here:
Distribution
Vertical Distribution
Off California trawling data show most vampires between depths of 600-1100 m with peaks at 700-800 m and 900-1000 m, and with small individuals of less than 20 mm being most abundant at the deeper peak (Roper and Young, 1975). ROV observations in Monterey Bay, California suggest that the vampire is restricted to the oxygen minimum layer in this bay at an average depth of 690 m and oxygen levels of 0.22 ml/l Hunt, 1996.

Vertical distribution chart modified from Clarke and Lu, 1975.
Off Hawaii, 10 of 11 captures came from depths of 800-1200 m but little towing was done in deeper water. Two captures were from opening-closing nets at depths of about 800-950 m.
In the Atlantic at 18° N, 25° W, the vampire shows a peak distribution between 700 and 1200 m but without a clear size/depth pattern (Clarke and Lu, 1975).
All captures were made with opening/closing trawls. Bars represent a capture and the bar length indicates the depth range of the trawl while open. Yellow bars indicate a daytime capture and blue bars a nighttime capture. Fishing effort between 1000-1250 m was about twice that between 1250 and 1500 m, and effort between 1000-1500 m was about 5 times that between 1500 and 2000 m and about the same as that between 500 and 1000 m.
Numerous records exists for captures in excess of 1200 m (e. g., see Roper and Young, 1975) from open nets. Unfortunately, due to the rather high probability of contamination from shallower depths, these records are of questionable value.
Horizontal Distribution
The vampire squid is broadly distributed throughout the depths of the world's tropical and temperate oceans. Some geographical variation has been noted. Young (1972) found that the beaks of vampire squid from the Pacific Ocean off California were distinctly smaller than those from vampires of the Gulf of Guinea in the Atlantic Ocean. He also noted differences in sucker size and gill size in vampires from these areas. Vampires from off Monterey, California have a predominance of reddish rather than black chromatophores.
References
Clarke, M. R. and C. C. Lu. 1975. Verical Distribution of Cephalopods at 18° N 25° W in the North Atlantic. Journal of the Marine Biological Association of the United Kingdom, 55 (1): 165-182.
Herring, P. J., P. N. Dilly and C. Cope 1994. The bioluminescent organs of the deep-sea cephalopod Vampyroteuthis infernalis (Cephalopoda: Vampyromorpha). J. Zool. Lond. 233: 45-55.
Hunt, J. C. 1996. The behavior and ecology of midwater cephalopods from Monterey Bay: Submersible and laboratory observations. Ph. D. Dissertation, Univ. Calif. Los Angeles. 231 pp.
Nesis, K. N. 1982. Abridged key to the cephalopod mollusks of the world's ocean. 385+ii pp. Light and Food Industry Publishing House, Moscow. (In Russian.). Translated into English by B. S. Levitov, ed. by L. A. Burgess (1987), Cephalopods of the world. T. F. H. Publications, Neptune City, NJ, 351pp.
Pickford, G. E. 1949. Vampyroteuthis infernalis Chun an archaic dibranchiate cephalopod. II. External anatomy. Dana-Report No. 32: 1-132.
Pickford, G. E. 1949. The distribution of the eggs of Vampyroteuthis infernalis Chun. Journal of Marine Research, 8(1):73-83.
Robison, B. H. 1995. Light in the ocean's midwaters. Scientific American 273:60-64.
Robison, B. H, K. R. Reisenbichler, J. C. Hunt and S. H. D. Haddock. 2003. Light production by the arm tips of the deep-sea cephalopod Vampyroteuthis infernalis. Biol. Bull., 205: 102-109.
Roper, C. F. E. and R. E. Young. 1975. Vertical distribution of pelagic cephalopods. Smithsonian Contributions to Zoolog, 209: 1-51.
Seibel, B. A., Thuesen, E. V. and Childress, J. J. 1998. Flight of the Vampire: Ontogenetic gait-transition in Vampyroteuthis infernalis (Cephalopoda: Vampyromorpha). J. exp. Biol. 201, 2413-2424.
Young, R. E. 1964. The anatomy of the vampire squid. Masters Thesis, Univ. of Southern Calif.
Young, R.E. 1967. Homology of retractile filaments of vampire squid. Science, 156(3782):1633-1634.
Young, R. E. 1972. The systematics and areal distribution of pelagic cephalopods from the seas off Southern California. Smithson. Contr. Zool., 97: 1-159.
Title Illustrations

| Scientific Name | Vampyroteuthis infernalis |
|---|---|
| Location | San Clemente Basin off Southern California |
| Comments | Photographed in plankton kreisel aboard the R/V NEW HORIZON. Captured at 700 m depth. |
| Size | 25 cm total length |
| Copyright | © 1999 Brad Seibel |
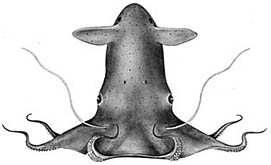
| Scientific Name | Vampyroteuthis infernalis |
|---|---|
| Reference | from Young, R. E. 1972. The systematics and areal distribution of pelagic cephalopods from the seas off Southern California. Smithson. Contr. Zool., 97: 1-159. |
| ToL Image Use |
 This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0. This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0.
|
| Copyright |
© Richard E. Young

|
About This Page
Richard E. Young

University of Hawaii, Honolulu, HI, USA
Page copyright © 1998
 Page: Tree of Life
Vampyromorpha . Vampyroteuthis infernalis . The Vampire Squid.
Authored by
Richard E. Young.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Vampyromorpha . Vampyroteuthis infernalis . The Vampire Squid.
Authored by
Richard E. Young.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
- Content changed 30 May 2008
Citing this page:
Young, Richard E. 2008. Vampyromorpha . Vampyroteuthis infernalis . The Vampire Squid. Version 30 May 2008. http://tolweb.org/Vampyroteuthis_infernalis/20084/2008.05.30 in The Tree of Life Web Project, http://tolweb.org/









 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site